
(a)
Interpretation:
To identify which of the given set of compounds have pKa value lesser than 20 and to identify the acidic proton in the same
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton
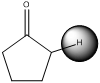
(a)
Answer to Problem 47PP
The pKa value of (a) is just below 20 and it has acidic proton next to carbonyl
Explanation of Solution
Deprotonation of the highlighted hydrogen and resonance stabilization
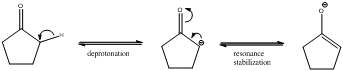
From the above scheme we can infer that the highlighted hydrogen is removed resulting in formation of a conjugate base. The conjugate base is resonance stabilized as the negative charge is delocalized on oxygen atom also. Therefore the highlighted proton is most acidic (pKa value below 20) as the removal of it leads to a stabilized conjugate base.
(b)
Interpretation:
To identify which of the given set of compounds have pKa value lesser than 20 and to identify the acidic proton in the same
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton
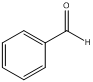
(b)
Answer to Problem 47PP
The pKa value of (b) is just above 20 and it does not have acidic proton
No acidic proton is present
Explanation of Solution
The above given compound does not have an acidic proton that can undergo deprotonation. Hence this compound is expected to have pKa above 20.
(c)
Interpretation:
To identify which of the given set of compounds have pKa value lesser than 20 and to identify the acidic proton in the same
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton

(c)
Answer to Problem 47PP
The pKa value of (c) is just below 20 and it has acidic proton next to carbonyl
Explanation of Solution
Deprotonation of the highlighted hydrogen and resonance stabilization
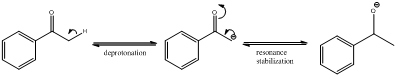
From the above scheme we can infer that the highlighted hydrogen is removed resulting in formation of a conjugate base. The conjugate base is resonance stabilized as the negative charge is delocalized on oxygen atom also. Therefore the highlighted proton is most acidic (pKa value below 20) as the removal of it leads to a stabilized conjugate base.
(d)
Interpretation:
To identify which of the given set of compounds have pKa value lesser than 20 and to identify the acidic proton in the same
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton

(d)
Answer to Problem 47PP
The pKa value of (d) is just below 20 and it has acidic proton next to carbonyl
Explanation of Solution
Deprotonation of the highlighted hydrogen and resonance stabilization
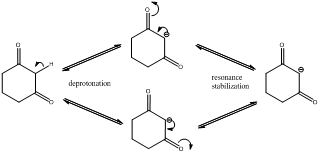
From the above scheme we can infer that the highlighted hydrogen is removed resulting in formation of a conjugate base. The conjugate base is resonance stabilized as the negative charge is delocalized on two oxygen atoms (doubly stabilized enolate ion). Therefore the highlighted proton is most acidic (pKa value below 20) as the removal of it leads to a stabilized conjugate base.
(e)
Interpretation:
To identify which of the given set of compounds have pKa value lesser than 20 and to identify the acidic proton in the same
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton

(e)
Answer to Problem 47PP
The pKa value of (e) is just below 20 and it has acidic proton next to carbonyl
Explanation of Solution
Deprotonation of the highlighted hydrogen

From the above scheme we can infer that the highlighted hydrogen is removed resulting in formation of an alokoxide ion. As such the compound is expected to have pKa lower than 20.
Want to see more full solutions like this?
Chapter 21 Solutions
ORGANIC CHEMISTRY 3E WPNGC LL SET 1S
- MnO2 acts as an oxidant in the chlorine synthesis reaction.arrow_forwardIn Potassium mu-dihydroxydicobaltate (III) tetraoxalate K4[Co2(C2O4)4(OH)2], indicate whether the OH ligand type is bidentate.arrow_forwardImagine an electrochemical cell based on these two half reactions with electrolyte concentrations as given below: Oxidation: Pb(s) → Pb2+(aq, 0.10 M) + 2 e– Reduction: MnO4–(aq, 1.50 M) + 4 H+(aq, 2.0 M) + 3 e– → MnO2(s) + 2 H2O(l) Calculate Ecell (assuming temperature is standard 25 °C).arrow_forward
- : ☐ + Draw the Fischer projection of the most common naturally-occurring form of aspartate, with the acid group at the top and the side chain at the bottom. Important: be sure your structure shows the molecule as it would exist at physiological pH. Click and drag to start drawing a structure. ✓arrow_forwardFor a silver-silver chloride electrode, the following potentials are observed: E°cell = 0.222 V and E(saturated KCl) = 0.197 V Use this information to find the [Cl–] (technically it’s the activity of Cl– that’s relevant here, but we’ll just call it “concentration” for simplicity) in saturated KCl.arrow_forwardA concentration cell consists of two Sn/Sn2+ half-cells. The cell has a potential of 0.10 V at 25 °C. What is the ratio of [Sn2+] (i.e., [Sn2+left-half] / [Sn2+right-half])?arrow_forward
- Electrochemical cell potentials can be used to determine equilibrium constants that would be otherwise difficult to determine because concentrations are small. What is Κ for the following balanced reaction if E˚ = +0.0218 V? 3 Zn(s) + 2 Cr3+(aq) → 3 Zn2+(aq) + Cr(s) E˚ = +0.0218 Varrow_forwardConsider the following half-reactions: Hg2+(aq) + 2e– → Hg(l) E°red = +0.854 V Cu2+(aq) + 2e– → Cu(s)E°red = +0.337 V Ni2+(aq) + 2e– → Ni(s) E°red = -0.250 V Fe2+(aq) + 2e– → Fe(s) E°red = -0.440 V Zn2+(aq) + 2e– → Zn(s) E°red = -0.763 V What is the best oxidizing agent shown above (i.e., the substance that is most likely to be reduced)?arrow_forwardCalculate the equilibrium constant, K, for MnO2(s) + 4 H+(aq) + Zn(s) → Mn2+(aq) + 2 H2O(l) + Zn2+(aq)arrow_forward
- In the drawing area below, draw the condensed structures of formic acid and ethyl formate. You can draw the two molecules in any arrangement you like, so long as they don't touch. Click anywhere to draw the first atom of your structure. A C narrow_forwardWrite the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule Ο C=O common name (not the IUPAC name) H ☐ H3N CH₂OH 0- C=O H NH3 CH₂SH H3N ☐ ☐ X Garrow_forward(Part A) Provide structures of the FGI products and missing reagents (dashed box) 1 eq Na* H* H -H B1 B4 R1 H2 (gas) Lindlar's catalyst A1 Br2 MeOH H2 (gas) Lindlar's catalyst MeO. OMe C6H1402 B2 B3 A1 Product carbons' origins Draw a box around product C's that came from A1. Draw a dashed box around product C's that came from B1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





