
(a)
To draw a PV diagram for the gas.
(a)
Answer to Problem 42PQ
The PV diagram for the gas is given below.
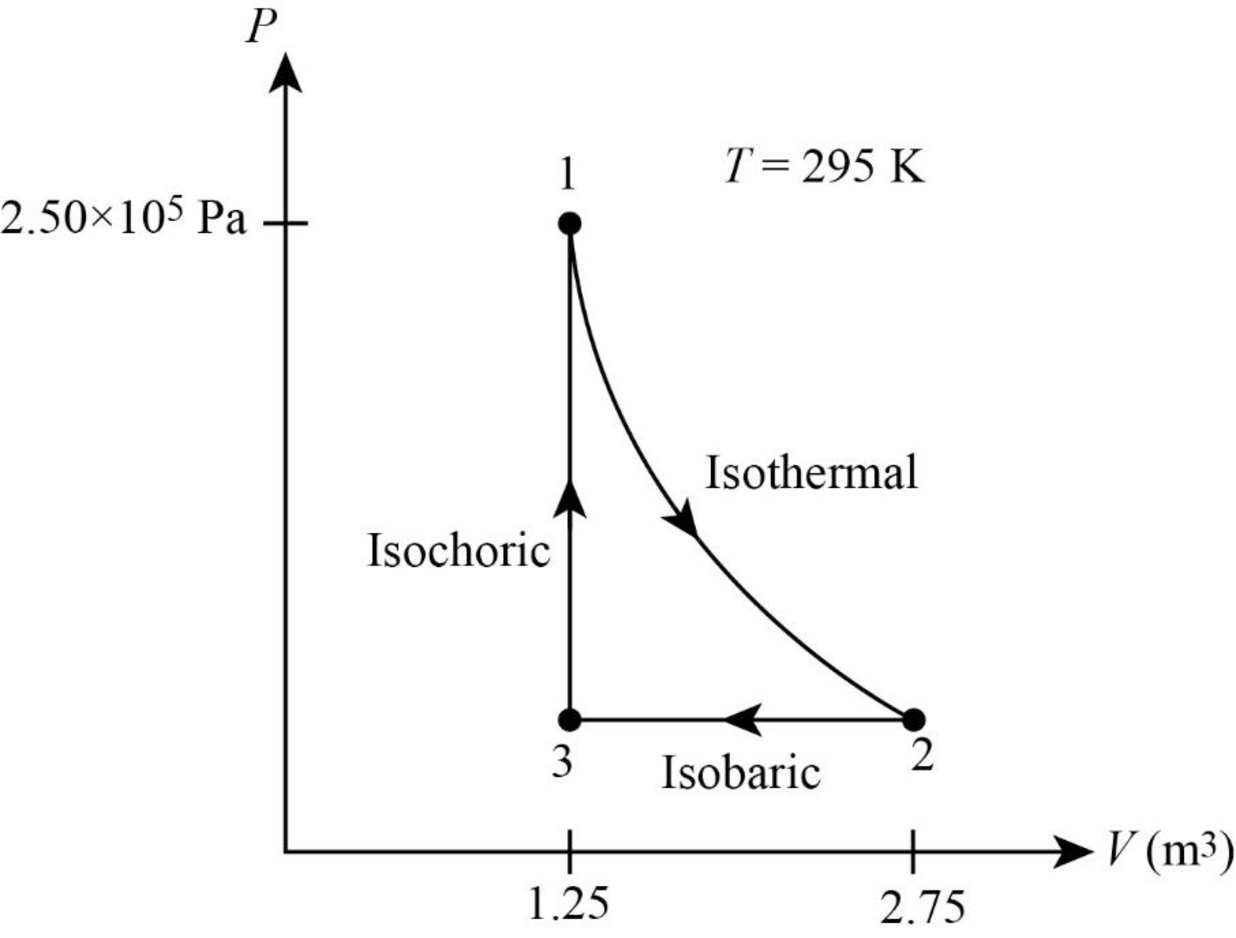
Explanation of Solution
It is given that pressure of the gas is
The following figure gives the PV diagram for the gas.
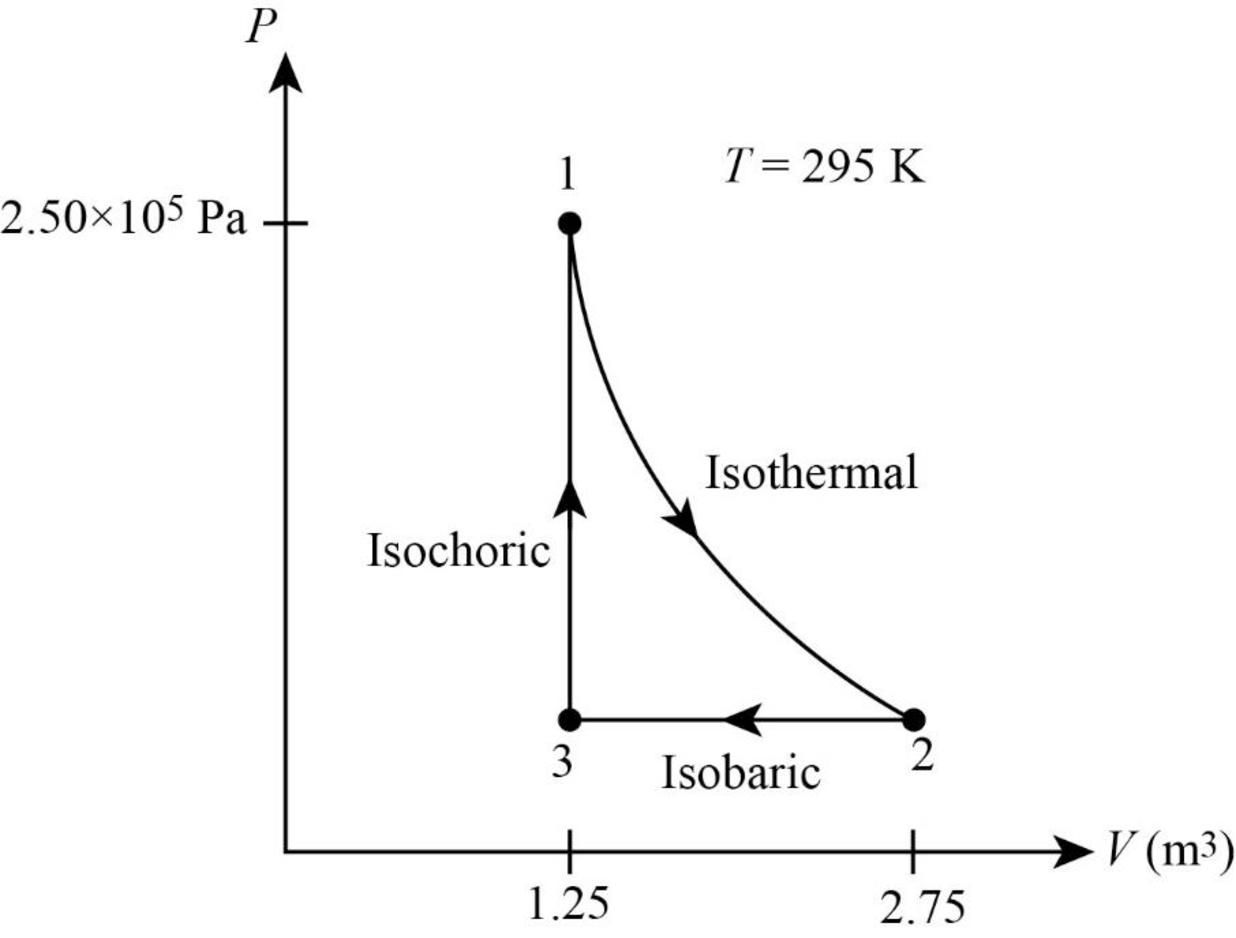
In above figure, point 1 represents the initial state. The gas follows isothermal expansion from
Conclusion:
Thus, the PV diagram for the gas is given below.
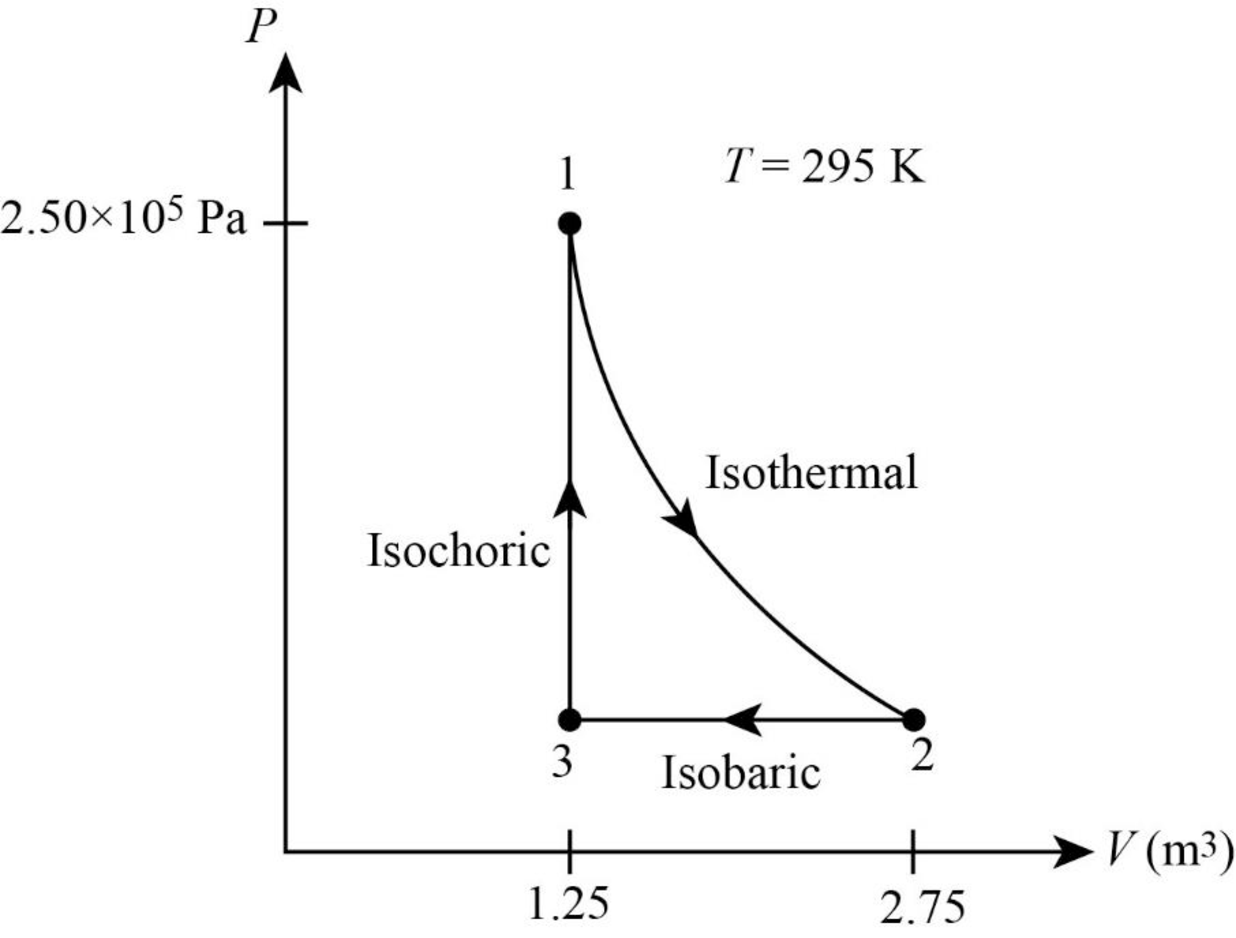
(b)
The change in thermal energy.
(b)
Answer to Problem 42PQ
The change in thermal energy of the gas is zero.
Explanation of Solution
From figure its clear that the gas undergoes a
In this problem it is given that the system return’s to its original state. Thus following a cyclic process, total change in thermal energy of the gas is zero.
Conclusion:
Therefore, the change in thermal energy of the gas is zero.
(c)
The work done by environment on the gas.
(c)
Answer to Problem 42PQ
The work done by environment on the gas is
Explanation of Solution
The net work done by the gas is the area inside the curve. In isothermal process volume of the system is raised from
Write the expression for the work done during isothermal expansion.
Here,
Write ideal gas equation.
Here,
Use above equation for the gas at initial state (state 1 in figure).
Here,
Substitute
For isothermal process,
Apply above equation for the isothermal process of the gas.
Here,
Rearrange above equation to get
In figure1, the straight line represents isobaric process where pressure is constant. During this process the system is compressed to original volume. Therefore, work is done on the system.
Write the expression for the work done in isobaric compression.
Here,
The negative sign indicates that work is done on the gas.
During isobaric process, the gas is at pressure
Use above equation to write work done by gas during isobaric compression shown in figure1.
Here,
In an isochoric process total work done is zero.
Write the expression for the total work done by the gas.
Here,
Conclusion:
Substitute
Substitute
Substitute
Substitute
Therefore, the work done by environment on the gas is
(d)
The heat that flows into the gas.
(d)
Answer to Problem 42PQ
The heat that flows into the gas is
Explanation of Solution
Write the first law of
Here,
Since the process is cyclic,
Substitute
Conclusion:
Substitute
Therefore, the heat that flows into the gas is
Want to see more full solutions like this?
Chapter 21 Solutions
EBK WEBASSIGN FOR KATZ'S PHYSICS FOR SC
- Hi Expert in Physics, Could you please Rewrite thses random equations using good formula of mathematics and explain each Greek alphabet and their meaning in English? Best Regards, Yahyaarrow_forwardHi Expert, I have uploaded picture, could you please name the Greek alphabet and their name in English?arrow_forwardHi Expert in Physics, I have uploaded pictures with respect to some physics equations. Could please name all Greek alphabet and their English name?arrow_forward
- 81 SSM Figure 29-84 shows a cross section of an infinite conducting sheet carrying a current per unit x-length of 2; the current emerges perpendicularly out of the page. (a) Use the Biot-Savart law and symmetry to show that for all points B P P. BD P' Figure 29-84 Problem 81. x P above the sheet and all points P' below it, the magnetic field B is parallel to the sheet and directed as shown. (b) Use Ampere's law to prove that B = ½µλ at all points P and P'.arrow_forwardWhat All equations of Ountum physics?arrow_forwardPlease rewrite the rules of Quantum mechanics?arrow_forward
- Suppose there are two transformers between your house and the high-voltage transmission line that distributes the power. In addition, assume your house is the only one using electric power. At a substation the primary of a step-down transformer (turns ratio = 1:23) receives the voltage from the high-voltage transmission line. Because of your usage, a current of 51.1 mA exists in the primary of the transformer. The secondary is connected to the primary of another step-down transformer (turns ratio = 1:36) somewhere near your house, perhaps up on a telephone pole. The secondary of this transformer delivers a 240-V emf to your house. How much power is your house using? Remember that the current and voltage given in this problem are rms values.arrow_forwardThe human eye is most sensitive to light having a frequency of about 5.5 × 1014 Hz, which is in the yellow-green region of the electromagnetic spectrum. How many wavelengths of this light can fit across a distance of 2.2 cm?arrow_forwardA one-dimensional harmonic oscillator of mass m and angular frequency w is in a heat bath of temperature T. What is the root mean square of the displacement of the oscillator? (In the expressions below k is the Boltzmann constant.) Select one: ○ (KT/mw²)1/2 ○ (KT/mw²)-1/2 ○ kT/w O (KT/mw²) 1/2In(2)arrow_forward
- Two polarizers are placed on top of each other so that their transmission axes coincide. If unpolarized light falls on the system, the transmitted intensity is lo. What is the transmitted intensity if one of the polarizers is rotated by 30 degrees? Select one: ○ 10/4 ○ 0.866 lo ○ 310/4 01/2 10/2arrow_forwardBefore attempting this problem, review Conceptual Example 7. The intensity of the light that reaches the photocell in the drawing is 160 W/m², when 0 = 18°. What would be the intensity reaching the photocell if the analyzer were removed from the setup, everything else remaining the same? Light Photocell Polarizer Insert Analyzerarrow_forwardThe lifetime of a muon in its rest frame is 2.2 microseconds. What is the lifetime of the muon measured in the laboratory frame, where the muon's kinetic energy is 53 MeV? It is known that the rest energy of the muon is 106 MeV. Select one: O 4.4 microseconds O 6.6 microseconds O 3.3 microseconds O 1.1 microsecondsarrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





