
(a)
Interpretation:
Electronic configuration of the following metals has to be written –
- (a) Ni (b) Cd (c) Zr (d) Os
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
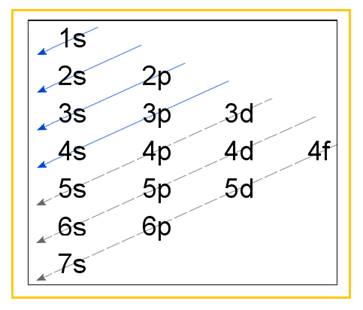
Figure 1
The terms
(b)
Interpretation:
Electronic configuration of the following metals has to be written –
- (a) Ni (b) Cd (c) Zr (d) Os
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
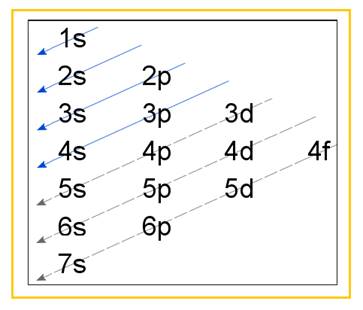
Figure 1
The terms
(c)
Interpretation:
Electronic configuration of the following metals has to be written –
- (a) Ni (b) Cd (c) Zr (d) Os
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
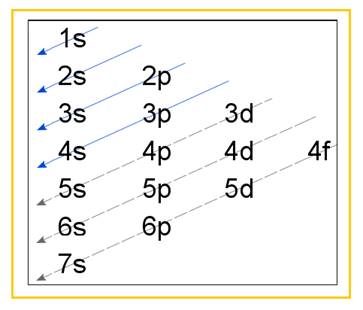
Figure 1
The terms
(d)
Interpretation:
Electronic configuration of the following metals has to be written –
- (a) Ni (b) Cd (c) Zr (d) Os
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
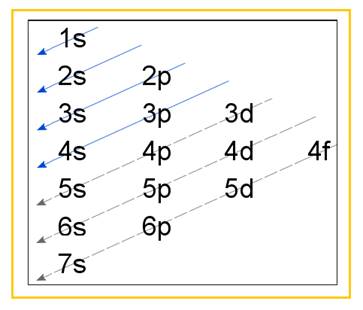
Figure 1
The terms
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
EBK CHEMISTRY
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





