
Bundle: General Chemistry, Loose-Leaf Version, 11th + LabSkills PreLabs v2 for Organic Chemistry (powered by OWLv2), 4 terms (24 months) Printed ... for Ebbing/Gammon's General Chemistry, 11th
11th Edition
ISBN: 9781337542630
Author: Darrell Ebbing, Steven D. Gammon
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.1, Problem 2.1CC
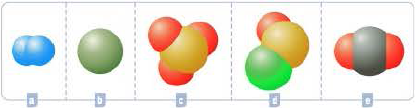
Like Dalton, chemists continue to model atoms using spheres. Modern models are usually drawn with a computer and use different colors to represent atoms of different elements. Which of the models below represents CO2?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution,
respectively.
F CI
Br |
Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to
have a reasonable yield of product.
NH2
Br
Br
Br
OH
Br
Q7: Rank the following groups in order of basicity, nucleophilicity, and leaving group ability.
a) H₂O, OH, CH3COOT
b) NH3, H₂O, H₂S
Q8: Rank the following compounds in order of increasing reactivity in a nucleophilic substitution
reaction with CN as the nucleophile.
Br
A
B
NH2
LL
F
C
D
OH
CI
LLI
E
Q9: Complete the missing entities for following reactions (e.g., major product(s), reactants,
and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for
reactions a) to d).
a)
H
"Cl
D
+
-OCH 3
Page 3 of 5
Chapter 2 Solutions
Bundle: General Chemistry, Loose-Leaf Version, 11th + LabSkills PreLabs v2 for Organic Chemistry (powered by OWLv2), 4 terms (24 months) Printed ... for Ebbing/Gammon's General Chemistry, 11th
Ch. 2.1 - Like Dalton, chemists continue to model atoms...Ch. 2.2 - Prob. 2.2CCCh. 2.3 - A nucleus consists of 17 protons and 18 neutrons....Ch. 2.4 - Chlorine consists of the following isotopes:...Ch. 2.5 - By referring to the periodic table (Figure 2.15 or...Ch. 2.5 - Consider the elements He, Ne, and Ar. Can you come...Ch. 2.6 - Prob. 2.4ECh. 2.6 - Classify each of the following as either an ionic...Ch. 2.8 - Prob. 2.5CCCh. 2.8 - Prob. 2.5E
Ch. 2.8 - Prob. 2.6ECh. 2.8 - Prob. 2.6CCCh. 2.8 - Prob. 2.7ECh. 2.8 - Prob. 2.8ECh. 2.8 - Prob. 2.9ECh. 2.8 - Prob. 2.10ECh. 2.8 - Washing soda has the formula Na2CO310H2O. What is...Ch. 2.8 - Prob. 2.12ECh. 2.8 - Prob. 2.7CCCh. 2.10 - Prob. 2.13ECh. 2 - Describe atomic theory and discuss how it explains...Ch. 2 - Two compounds of iron and chlorine, A and B,...Ch. 2 - Explain the operation of a cathode-ray tube....Ch. 2 - Prob. 2.4QPCh. 2 - Prob. 2.5QPCh. 2 - What are the different kinds of particles in the...Ch. 2 - Describe how protons and neutrons were discovered...Ch. 2 - Oxygen consists of three different _____, each...Ch. 2 - Describe how Dalton obtained relative atomic...Ch. 2 - Briefly explain how a mass spectrometer works....Ch. 2 - Define the term atomic weight. Why might the...Ch. 2 - What is the name of the element in Group 4A and...Ch. 2 - Prob. 2.13QPCh. 2 - Prob. 2.14QPCh. 2 - Prob. 2.15QPCh. 2 - What is the fundamental difference between an...Ch. 2 - Prob. 2.17QPCh. 2 - Which of the following models represent a(n): a...Ch. 2 - Prob. 2.19QPCh. 2 - Prob. 2.20QPCh. 2 - How many protons, neutrons, and electrons are in...Ch. 2 - The atomic weight of Ga is 69.72 amu. There are...Ch. 2 - Prob. 2.23QPCh. 2 - A chunk of an unidentified element (lets call it...Ch. 2 - Average Atomic Weight Part 1: Consider the four...Ch. 2 - Model of the Atom Consider the following...Ch. 2 - One of the early models of the atom proposed that...Ch. 2 - A friend is trying to balance the following...Ch. 2 - Given that the periodic table is an organizational...Ch. 2 - Prob. 2.30QPCh. 2 - Prob. 2.31QPCh. 2 - Match the molecular model with the correct...Ch. 2 - Consider a hypothetical case in which the charge...Ch. 2 - Prob. 2.34QPCh. 2 - Prob. 2.35QPCh. 2 - You perform a chemical reaction using the...Ch. 2 - Prob. 2.37QPCh. 2 - Prob. 2.38QPCh. 2 - Prob. 2.39QPCh. 2 - Prob. 2.40QPCh. 2 - A student has determined the mass-to-charge ratio...Ch. 2 - The mass-to-charge ratio for the positive ion F+...Ch. 2 - The following table gives the number of protons...Ch. 2 - The following table gives the number of protons...Ch. 2 - Naturally occurring chlorine is a mixture of the...Ch. 2 - Naturally occurring nitrogen is a mixture of 14N...Ch. 2 - What is the nuclide symbol for the nucleus that...Ch. 2 - An atom contains 34 protons and 45 neutrons. What...Ch. 2 - Ammonia is a gas with a characteristic pungent...Ch. 2 - Hydrogen sulfide is a gas with the odor of rotten...Ch. 2 - Calculate the atomic weight of an element with two...Ch. 2 - An element has two naturally occurring isotopes...Ch. 2 - An element has three naturally occurring isotopes...Ch. 2 - An element has three naturally occurring isotopes...Ch. 2 - While traveling to a distant universe, you...Ch. 2 - While roaming a parallel universe, you discover...Ch. 2 - Identify the group and period for each of the...Ch. 2 - Prob. 2.58QPCh. 2 - Prob. 2.59QPCh. 2 - Prob. 2.60QPCh. 2 - Prob. 2.61QPCh. 2 - Prob. 2.62QPCh. 2 - The normal form of the element sulfur is a...Ch. 2 - White phosphorus is available in sticks, which...Ch. 2 - A 4.19-g sample of nitrous oxide (an anesthetic,...Ch. 2 - Nitric acid is composed of HNO3 molecules. A...Ch. 2 - A sample of ammonia, NH3, contains 1.2 1023...Ch. 2 - A sample of ethanol (ethyl alcohol), C2H3OH,...Ch. 2 - Prob. 2.69QPCh. 2 - What molecular formula corresponds to each of the...Ch. 2 - Prob. 2.71QPCh. 2 - Prob. 2.72QPCh. 2 - Prob. 2.73QPCh. 2 - Ammonium phosphate, (NH4)3PO4, has how many oxygen...Ch. 2 - Prob. 2.75QPCh. 2 - Prob. 2.76QPCh. 2 - Name the following compounds. a Na2SO4 b Na3N c...Ch. 2 - Name the following compounds. a CaO b Mn2O3 c...Ch. 2 - Prob. 2.79QPCh. 2 - Prob. 2.80QPCh. 2 - Prob. 2.81QPCh. 2 - For each of the following binary compounds, decide...Ch. 2 - Give systematic names to the following binary...Ch. 2 - Prob. 2.84QPCh. 2 - Prob. 2.85QPCh. 2 - Prob. 2.86QPCh. 2 - Prob. 2.87QPCh. 2 - Prob. 2.88QPCh. 2 - Prob. 2.89QPCh. 2 - Give the name and formula of the acid...Ch. 2 - Prob. 2.91QPCh. 2 - Prob. 2.92QPCh. 2 - Prob. 2.93QPCh. 2 - Prob. 2.94QPCh. 2 - For the balanced chemical equation Ca(NO3)2 +...Ch. 2 - In the equation 2PbS + O2 2PbO + 2SO2, how many...Ch. 2 - Balance the following equations. a Sn + NaOH ...Ch. 2 - Balance the following equations. a Ca3(PO4)2 +...Ch. 2 - Prob. 2.99QPCh. 2 - Solid sodium metal reacts with water, giving a...Ch. 2 - Prob. 2.101QPCh. 2 - Prob. 2.102QPCh. 2 - Two samples of different compounds of nitrogen and...Ch. 2 - Two samples of different compounds of sulfur and...Ch. 2 - In a series of oil-drop experiments, the charges...Ch. 2 - In a hypothetical universe, an oil-drop experiment...Ch. 2 - Compounds of europium. Eu, are used to make color...Ch. 2 - Cesium, Cs, is used in photoelectric cells...Ch. 2 - Prob. 2.109QPCh. 2 - One isotope of a metallic element has mass number...Ch. 2 - Obtain the fractional abundances for the two...Ch. 2 - Silver has two naturally occurring isotopes, one...Ch. 2 - Prob. 2.113QPCh. 2 - Prob. 2.114QPCh. 2 - Prob. 2.115QPCh. 2 - Prob. 2.116QPCh. 2 - Prob. 2.117QPCh. 2 - Prob. 2.118QPCh. 2 - Name the following compounds. a Sn3(PO4)2 b NH4NO2...Ch. 2 - Name the following compounds. a Cu(NO2)3 b (NH4)3P...Ch. 2 - Prob. 2.121QPCh. 2 - Prob. 2.122QPCh. 2 - Prob. 2.123QPCh. 2 - Name the following molecular compounds a ClF4 b...Ch. 2 - Prob. 2.125QPCh. 2 - Balance the following equations. a NaOH + H2CO3 ...Ch. 2 - A monatomic ion has a charge of +4. The nucleus of...Ch. 2 - A monatomic ion has a charge of +1. The nucleus of...Ch. 2 - Natural carbon, which has an atomic weight of...Ch. 2 - A sample of natural chlorine, has an atomic weight...Ch. 2 - Prob. 2.131QPCh. 2 - Prob. 2.132QPCh. 2 - Prob. 2.133QPCh. 2 - Prob. 2.134QPCh. 2 - Prob. 2.135QPCh. 2 - Ammonia gas reacts with molecular oxygen gas to...Ch. 2 - A hypothetical element X is found to have an...Ch. 2 - A monotomic ion has a charge of +3. The nucleus of...Ch. 2 - A small crystal of CaCl2 that weighs 0.12 g...Ch. 2 - Prob. 2.140QPCh. 2 - Prob. 2.141QPCh. 2 - The IO3, anion is called iodate. There are three...Ch. 2 - Prob. 2.143QPCh. 2 - From the following written description, write the...Ch. 2 - Prob. 2.145QPCh. 2 - Name the following compounds: a HCl(g) b HBr(aq) c...Ch. 2 - During nuclear decay a 238U atom can break apart...Ch. 2 - Prob. 2.148QPCh. 2 - There are 2.619 1022 atoms in 1.000 g of sodium....Ch. 2 - There are 1.699 1022 atoms in 1.000 g of...Ch. 2 - A sample of green crystals of nickel(II) sulfate...Ch. 2 - Cobalt(II) sulfate heptahydrate has pink-colored...Ch. 2 - A sample of metallic element X, weighing 3.177 g,...Ch. 2 - A sample of metallic element X, weighing 4.315 g,...Ch. 2 - Prob. 2.156QPCh. 2 - The element europium exists in nature as two...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify me theme or themes exemplified by (a) the sharp quills of a porcupine (b) the development of a multice...
Campbell Biology in Focus (2nd Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
- Explain why only the lone pairs on the central atom are taken into consideration when predicting molecular shapearrow_forward(ME EX1) Prblm #9/10 Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.arrow_forwardProblems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY