
Concept explainers
Give the IUPAC name for each compound.
a. 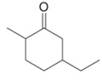 c.
c. 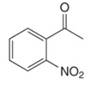 e.
e. 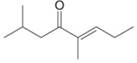
b. 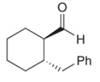 d.
d. 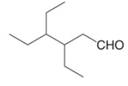 f.
f. 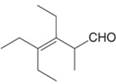
(a)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of six
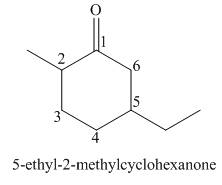
Figure 1
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(b)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
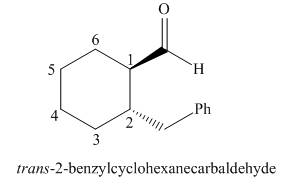
Figure 2
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(c)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of six
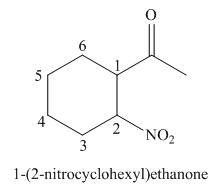
Figure 3
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(d)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
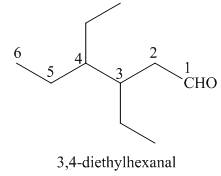
Figure 4
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(e)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of eight
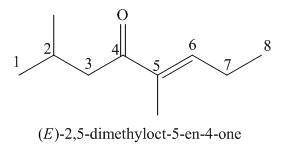
Figure 5
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(f)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
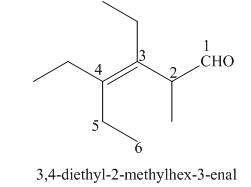
Figure 6
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
Want to see more full solutions like this?
Chapter 21 Solutions
PKG ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Loose Leaf For Integrated Principles Of Zoology
Biology: Concepts and Investigations
Brock Biology of Microorganisms (15th Edition)
General, Organic, and Biological Chemistry - 4th edition
- Search Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forwardConsider the reaction below to answer the following questions: Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 1. NaOEt, EtOH H&C OCH CH3 2 H30 H3C CH2 OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. OEtarrow_forwardUse the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 32 16 solid liquid gas 0 0 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Шос ☑ كarrow_forward
- Starting from bromoethane, how could you prepare the following compounds: a. Ethanol. b. Acetaldehyde f. Acetone. e. 2-Propanol i. Acetoacetic ester. d. 2-Bromoacetic acid. c. Acetic acid g. Acetamide. j. Ethylmalonate k. Gama ketoacid. h. Ethyl magnesium bromide.arrow_forward- The pressure above a pure sample of solid Substance X at 60. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 02 0.4 solid Hliquid gas 0 0 200 400 600 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. ☐ atmarrow_forward15. What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 0 O H3C COC CH3 H₂C C N(CH3)2 H3C C OCH3 A. a. I, 11, 111, b. I, III, IV, II C. II, IV, III, I ° (CH3)2CH C OCH3 IV d. II, I, III, IV B. R COCR 0 0 0 13= RC NH2 RC OR RC CI === IV a. I, III, II, IV b. II, III, I, IV C. III, II, I, IV d. IV, I, III, IIarrow_forward
- Draw the formula of the product obtained by reacting D-Tallose with bromine water.arrow_forwardChoose the best reagent(s) for carrying out the following conversions from the list below. Place the letter corresponding to the best choice in the blank to the left of the conversion. a. KMnO4, H3O+ b. Tollens' Reagent [oxidizing reagent] C. NaBH4, ethanol d. 1. BH3 2. H3O+ e. 1. CH3MgBr, ether 2. H3O+ f. CrO3, H2SO4, H₂O g. 1. Mg, ether 2. CO2 3. H3O+ h. 1. NaCN 2. H2SO4, H2, heat i. O3, then Zn and HOAC j. CH₂I A. B. C. CH CH=CHCH2COOH Br CEN CH COOH + HOOCCH COOH COOH 010 CH3arrow_forwardDraw the structures for each of the intermediates in the boxes provided for the synthesis below. OCH3 Fe HO HNO (CHOO pynding H₂504 LHNO2 NACH-I Fa H₂O HCL HNO 180arrow_forward
- Provide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry [three only] A. o 11 (CH3)CH — C— C ether (CH3)2CH-C-O-C-CH3 B. CH3 CHy CI Staf OH C. HC OCHS + H₂Oarrow_forwardConsider the reaction sequence below to answer the following questions: EtO Compound X 1. NaOEt, EtOH OEt Br CO₂Et NaOEt, EtOH Compound Z CO₂Et Compound Y A. Compound X, diethyl propanedioate, is more commonly known as a. ethyl acetoacetate b. acetoacetic ester C. oxalic ester d. malonic ester 3. Write the complete stepwise mechanism for the conversion of Compound X into Compound Y. Show all electron flow with arrows and draw all intermediate structures.arrow_forwardClassify each of the following nitrogen atoms in the following compounds as primary, secondary, tertiary, or quaternary [three only] CH3 HO-CHCHNHCH3 A. B. C. H&C CH3 D. HO phedrine CH2CHCH3 amphetamine NH₂ mepiquat chloride faxofenadine OH H&C CH CO₂Harrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



