
(a)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
The ester is hydrolyzed to the
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
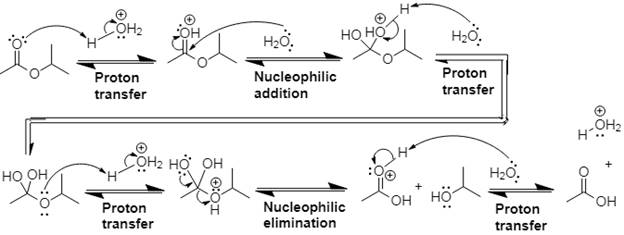
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed ester hydrolysis.
(b)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid-catalyzed transesterification, and a new ester is formed.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
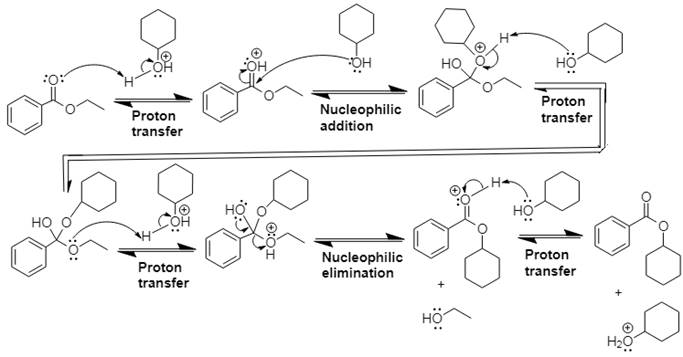
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2,
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed transesterification.
(c)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed amide hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
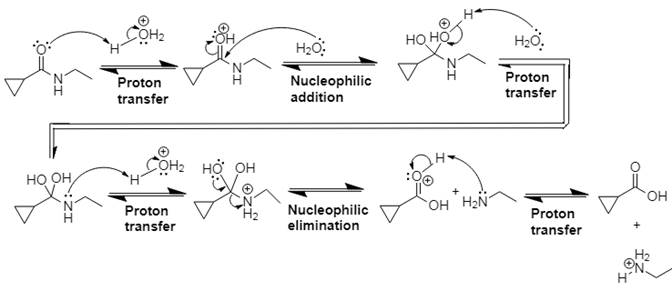
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed amide hydrolysis.
(d)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed ester hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of alcohol. Proton transfer steps are incorporated to avoid the appearance of strong bases.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
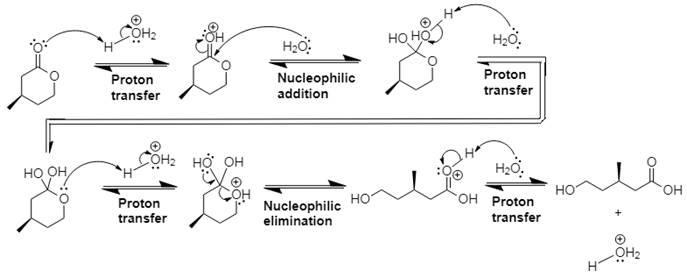
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed ester hydrolysis.
(e)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is Fischer esterification reaction.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
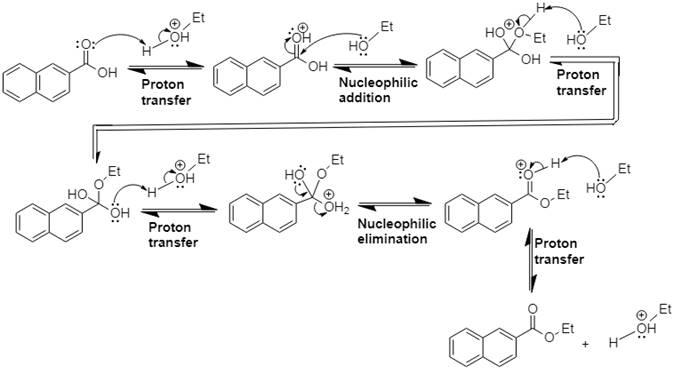
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the acid’s carbonyl group, and in Step 2,
The complete, detailed mechanism and the overall product for the given reaction is drawn by Fischer esterification reaction.
(f)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed amide hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of amine. Proton transfer steps are incorporated to avoid the appearance of strong bases.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
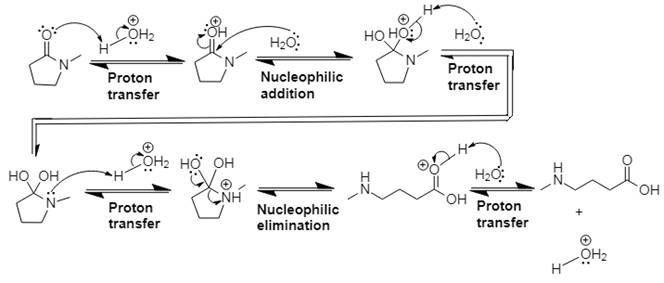
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed amide hydrolysis.
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry: Principles And Mechanisms
- Predict the major organic product(s) of the following reactions. Indicate which of the following mechanisms is in operation: SN1, SN2, E1, or E2.arrow_forward(c) (4pts) Mechanism: heat (E1) CH3OH + 1.5pts each _E1 _ (1pt) Br CH3OH (d) (4pts) Mechanism: SN1 (1pt) (e) (3pts) 1111 I H 10 Ill!! H LDA THF (solvent) Mechanism: E2 (1pt) NC (f) Bri!!!!! CH3 NaCN (3pts) acetone Mechanism: SN2 (1pt) (SN1) -OCH3 OCH3 1.5pts each 2pts for either product 1pt if incorrect stereochemistry H Br (g) “,、 (3pts) H CH3OH +21 Mechanism: SN2 (1pt) H CH3 2pts 1pt if incorrect stereochemistry H 2pts 1pt if incorrect stereochemistryarrow_forwardA mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixturearrow_forward
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
