
Concept explainers
(a)
To draw: The circle on isoprene unit in the astaxanthin molecule.
Introduction:
Isoprenoids are the natural occurring precursor of cholesterol and contain a huge variety. Isoprenoids are synthesized by the mevalonate pathway and are commercially and medically important. Their synthetic production is a low yielding expensive process.
Isoprenoids are the precursors of astaxanthin, which is a type of carotenoid. It appears red orange in color. It serves as a strong antioxidant that can be absorbed by humans.
(a)
Explanation of Solution
Pictorial representation: Fig.1 shows isoprene unit in astaxanthin molecule.
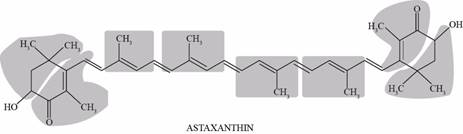
Fig.1: Isoprene unit in astaxanthin molecule.
Explanation:
Astaxanthin molecule is made up of eight isoprene molecules. It is a type of carotenoid which occurs in salmon, algae, shrimp, and lobster, and appears orange-red in color.
(b)
To determine: Whether two molecules of geranylgeranyl pyrophosphate are joined head to head or head to tail.
Introduction:
Phyotene is an intermediate formed during the synthesis of astaxanthin and other carotenoid molecule. Its function is to maintain the growth and development of plastids.
(b)
Explanation of Solution
Pictorial representation: Fig.2 shows the Phyotene.
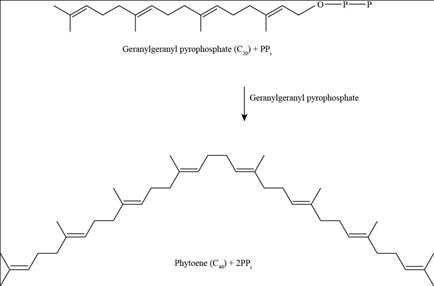
Fig.2: Phyotene.
Explanation:
Phyotene molecules are formed by “head-to-head joining” of two geranylgeranyl pyrophosphate molecules, as phyotene contain dimethyl group at the tail position. The joining of two geranylgeranyl pyrophosphate molecules releases diphosphate during joining without releasing a free -OH group.
(c)
To determine: The chemical transformation during the production in step five of astaxanthin synthesis.
Introduction:
Phyotene is an intermediate formed during the synthesis of astaxanthin and other carotenoid molecule. Its function is to maintain the growth and development of plastids.
(c)
Explanation of Solution
Explanation:
In chemical transformation during fifth step, phyotene is converted into lycopene. The enzyme dehydrogenase is responsible for catalyzing the transformation reaction and it converts four single bonds into double bond.
(d)
To determine: The requirement of net oxidation for cyclization during cholesterol synthesis.
Introduction:
Cholesterol consists of four ring structure which is fused together. It contains two methyl groups which lie above the plain containing four rings. Cholesterol is a component of plasma protein and plasma membrane, and present in abundance in nerve tissues
(d)
Explanation of Solution
Explanation:
There is no requirement of net oxidation for cyclization during cholesterol synthesis as during this process, two single bonds are replaced by double bond, hence there is no oxidation or reduction.
(e)
To determine: The step in the pathway which is catalyzed by the enzyme encoded by gps gene
Introduction:
Two geranylgeranyl pyrophosphate molecules are joined together and results in the formation of phyotene. The reaction releases diphosphate during joining without releasing a free –OH group
(e)
Explanation of Solution
Explanation:
Gene gps encodes the enzyme which regulates the steps one to three in the pathway. The pathway represents the conversion of
(f)
To determine: The expression level of the enzyme which is capable of catalyzing step 3 of the astaxanthin synthesis pathway.
Introduction:
Isoprenoids are the precursors of astaxanthin, which is a type of carotenoid, and appear red orange in color. It serves as a strong antioxidant that can be absorbed by humans.
(f)
Explanation of Solution
Explanation:
The comparison of strain1 through strain 4 does not express astaxanthin, whereas strain 5 through strain 8 shows over expression of gene crtE and leads to overproduction astaxanthin.
Third step of astaxanthin involves the conversion of farnesyl pyrophosphate into geranylgeranyl pyrophosphate, in wild type E. coli strain.
(g)
To determine: The enzyme which is rate limiting in the astaxanthin synthesis pathway.
Introduction:
In the astaxanthin synthesis pathway in E. coli, enzymes are encoded by crtBIZYW, crtE, ispA and idi genes, which catalyze the pathway and yield astaxanthin. The idi encodes for IPP isomerase.
(g)
Explanation of Solution
Explanation:
The ispA gene catalyzes step one and two of the pathway in strain 5 and 6 and results in a little increase in the production of astaxanthin. idi gene catalyzes the process in the strain 7 and strain 8 and shows increase in the production of astaxanthin. Hence, the enzyme IPP isomerase catalyzes the rate limiting step of the pathway when the gene crtE is overexpressed.
(h)
To determine: Whether a strain overexpressing crtBIZYW, gps and crtE genes will produce low (+), medium (++), or high (+++) levels of astaxanthin, as measured by its orange color
Introduction:
In the astaxanthin synthesis pathway in E. coli, enzymes are encoded by crtBIZYW, crtE, ispA and idi genes, which catalyze the pathway and yield astaxanthin. Idi encodes for IPP isomerase.
(h)
Explanation of Solution
Explanation:
crtBIZYW, gps and crtE genes are expressed in strain 5, strain 6, and strain 9 and produce a low level of astaxanthin, The genes expressed in strain 7, strain 8 and strain 9 overproduces astaxanthin because of over expression of idi gene. Thus, IPP isomeraselimits the production of astaxanthin.
Want to see more full solutions like this?
Chapter 21 Solutions
SAPLINGPLUS FOR PRINCIPLES OF BIOCHEMIS
- The following data were recorded for the enzyme catalyzed conversion of S -> P Question: what would the rate be at 5.0 x 10-5 M [S] and the enzyme concentration was doubled? Also, the rate given in the table is from product accumulation after 10 minuets of reaction time. Verify these rates represent a true initial rate (less than 5% turnover). Please helparrow_forwardThe following data was obtained on isocitrate lyase from an algal species. Identify the reaction catalyzed by this enzyme, deduce the KM and Vmax , and determine the nature of the inhibition by oxaloacetate. Please helparrow_forwardIn the table below, there are sketches of four crystals made of positively-charged cations and negatively-charged anions. Rank these crystals in decreasing order of stability (or equivalently increasing order of energy). That is, select "1" below the most stable (lowest energy) crystal. Select "2" below the next most stable (next lowest energy) crystal, and so forth. A B 鹽 (Choose one) +2 C +2 +2 (Choose one) D 鹽雞 (Choose one) (Choose one)arrow_forward
- 1. Draw the structures for the fats A. 16:2: w-3 and B. 18:3:49,12,15 2. Name each of the molecules below (image attached)arrow_forwarddraw the structures for the fats A. 16:2:w-3 B 18:3:9,12,15arrow_forward1. Below is a template strand of DNA. Show the mRNA and protein that would result. label the ends of the molecules ( refer to attached image)arrow_forward
- Attach the followina labels to the diagram below: helicase, single stranded binding proteins, lagging strand, leading strand, DNA polymerase, primase, 5' ends (3), 3' ends (3) (image attached)arrow_forward1. How much energy in terms of ATP can be obtained from tristearin (stearate is 18:0) Show steps pleasearrow_forwardMultiple choice urgent!!arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





