
Concept explainers
(a)
The temperature of the gas at B if the temperature at A is
(a)
Answer to Problem 51SP
Solution:
Explanation of Solution
Given data:
Refer to Fig. 20-6.
The temperature at A is
The mass of gas enclosed in the cylinder is
The gas follows the process A to B in the thermodynamic cycle shown in Fig. 20-6.
Formula used:
The gas equation for a process is expressed as
Here,
The formula for the conversion of the initial temperature of a gas from the Celsius scale to the Kelvin scale is
Here,
Explanation:
Draw the thermodynamic cycle diagram given in Fig- 20.6:
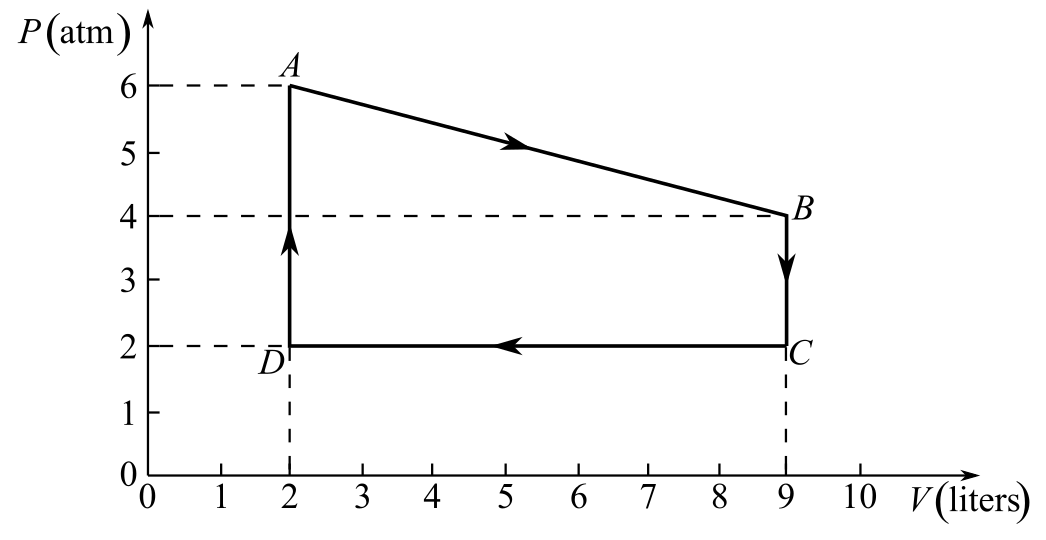
Recall the expression for the conversion of temperature at A from Celsius to Kelvin:
Here,
Substitute
Refer to the diagram and write the values of pressure and volume at points A and B, respectively,
And
Here,
Recall the gas equation between points A and B:
Here,
Substitute
Conclusion:
The temperature at point B is
(b)
The value of
(b)
Answer to Problem 51SP
Solution:
Explanation of Solution
Given data:
Refer to Fig. 20-6.
The temperature at A is
The heat received by the gas from A to B is
The mass of the gas enclosed in the cylinder is
Formula used:
The area of a trapezium is calculated by the formula:
Here,
The work done in a thermodynamic process is given by the area under the line representing the process in the pressure–volume diagram:
Here,
The first law of thermodynamics for a process is written as
Here,
The formula for change in internal energy is
Here,
The formula for conversion of temperature of gas from Kelvin scale to Celsius scale is
Here,
Explanation:
Draw the thermodynamic cycle diagram given in Fig- 20.6:

Understand that the work done in the thermodynamic process AB is equal to the area of the pressure–volume diagram under the line AB.
Draw the thermodynamic cycle diagram showing the area under the line AB:
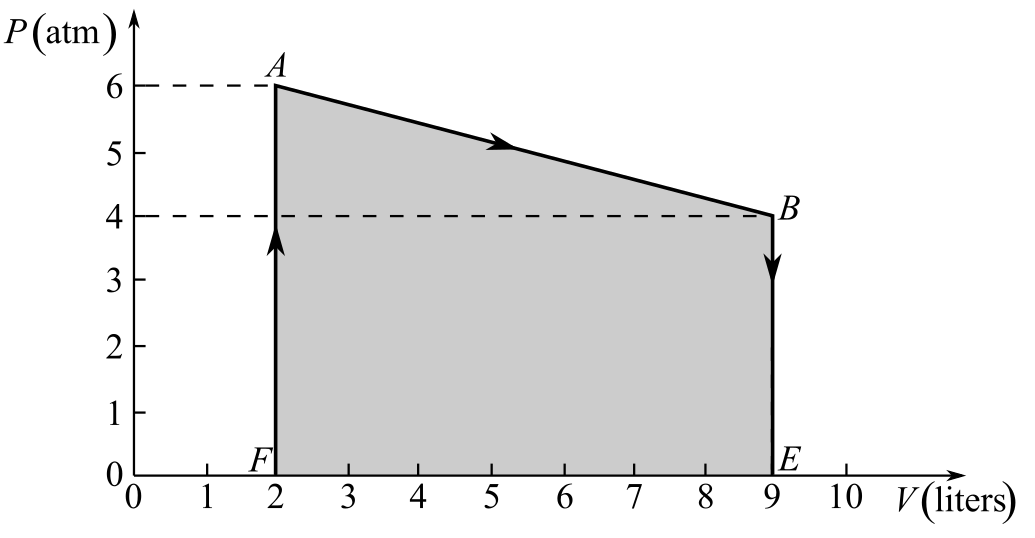
Here, the points E and F are as shown in the figure and the work done during the process AB is represented by the area under the line AB, which is equal to the area of the trapezium ABEF.
Refer to the figure and write the values of the lengths of sides AF, BE, and EF:
Recall the expression for the area of trapezium ABEF to calculate the area under the line AB in order to calculate the work done in the process AB:
Here,
Substitute
Recall the expression for the net-work done in a thermodynamic process in terms of the area of the pressure–volume diagram:
Substitute
Recall the expression for the first law of thermodynamics for the process AB:
According to the problem, the heat supplied from A to B is
Substitute
Further solve as
Calculate the temperature at point B in Celsius:
Here,
Substitute
Calculate the change in temperature from A to B:
Substitute
Recall the formula for change in internal energy:
Substitute
Conclusion:
The value of
Want to see more full solutions like this?
Chapter 20 Solutions
Schaum's Outline of College Physics, Twelfth Edition (Schaum's Outlines)
- need help part a and barrow_forwardComplete the table below for spherical mirrors indicate if it is convex or concave. Draw the ray diagrams S1 10 30 S1' -20 20 f 15 -5 Marrow_forwardA particle with a charge of − 5.20 nC is moving in a uniform magnetic field of (B→=−( 1.22 T )k^. The magnetic force on the particle is measured to be(F→=−( 3.50×10−7 N )i^+( 7.60×10−7 N )j^. Calculate the scalar product v→F→. Work the problem out symbolically first, then plug in numbers after you've simplified the symbolic expression.arrow_forward
- Need help wity equilibrium qestionarrow_forwardneed answer asap please thanks youarrow_forwardA man slides two boxes up a slope. The two boxes A and B have a mass of 75 kg and 50 kg, respectively. (a) Draw the free body diagram (FBD) of the two crates. (b) Determine the tension in the cable that the man must exert to cause imminent movement from rest of the two boxes. Static friction coefficient USA = 0.25 HSB = 0.35 Kinetic friction coefficient HkA = 0.20 HkB = 0.25 M₁ = 75 kg MB = 50 kg P 35° Figure 3 B 200arrow_forward
- A golf ball is struck with a velocity of 20 m/s at point A as shown below (Figure 4). (a) Determine the distance "d" and the time of flight from A to B; (b) Determine the magnitude and the direction of the speed at which the ball strikes the ground at B. 10° V₁ = 20m/s 35º Figure 4 d Barrow_forwardThe rectangular loop of wire shown in the figure (Figure 1) has a mass of 0.18 g per centimeter of length and is pivoted about side ab on a frictionless axis. The current in the wire is 8.5 A in the direction shown. Find the magnitude of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane. Find the direction of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane.arrow_forwardA particle with a charge of − 5.20 nC is moving in a uniform magnetic field of (B→=−( 1.22 T )k^. The magnetic force on the particle is measured to be (F→=−( 3.50×10−7 N )i^+( 7.60×10−7 N )j^. Calculate the y and z component of the velocity of the particle.arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College





