
(a)
Interpretation:
The missing element needs to be determined. The type of decay needs to be identified.
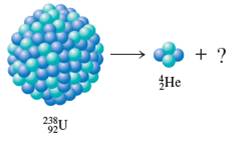
Concept Introduction:
Any unstable atomic nucleus would always convert to smaller stable fragments and this is termed as radioactive decay. When there is unbalance of protons and neutrons in the nucleus, then decay happens releasing energy in the form of radiations. These radiations are alpha, beta and gamma.
(b)
Interpretation:
The missing element needs to be determined. The type of decay needs to be identified.
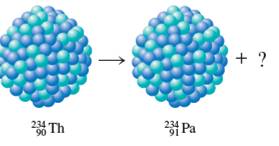
Concept Introduction:
Radioactive decays involve the conversion of unstable atomic nucleus to smaller stable fragments. Here it is an unbalance of protons and neutrons in the nucleus, which releases energy in the form of radiations. These radiations are alpha, beta and gamma.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
EBK CHEMICAL PRINCIPLES
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





