
Concept explainers
A vessel contains 1.00 × 104 oxygen molecules at 500 K. (a) Make an accurate graph of the Maxwell speed distribution function versus speed with points at speed intervals of 100 m/s. (b) Determine the most probable speed from this graph. (c) Calculate the average and rms speeds for the molecules and label these points on your graph. (d) From the graph, estimate the fraction of molecules with speeds in the range 300 m/s to 600 m/s.
(a)
The graph of the Maxwell speed distribution function versus speed with points at speed intervals of
Answer to Problem 38AP
The of the Maxwell speed distribution function versus speed with points at speed intervals of
Explanation of Solution
The Maxwell distribution curve is the graph between the distribution of speed and the change in speed or speed interval.
The number of molecules of oxygen in vessel is
Write the expression of Maxwell’s speed distribution function.
Here,
The mass of the molecules of oxygen
Here,
The molecular mass of the oxygen molecules in
Substitute
Substitute
Substitute the values of
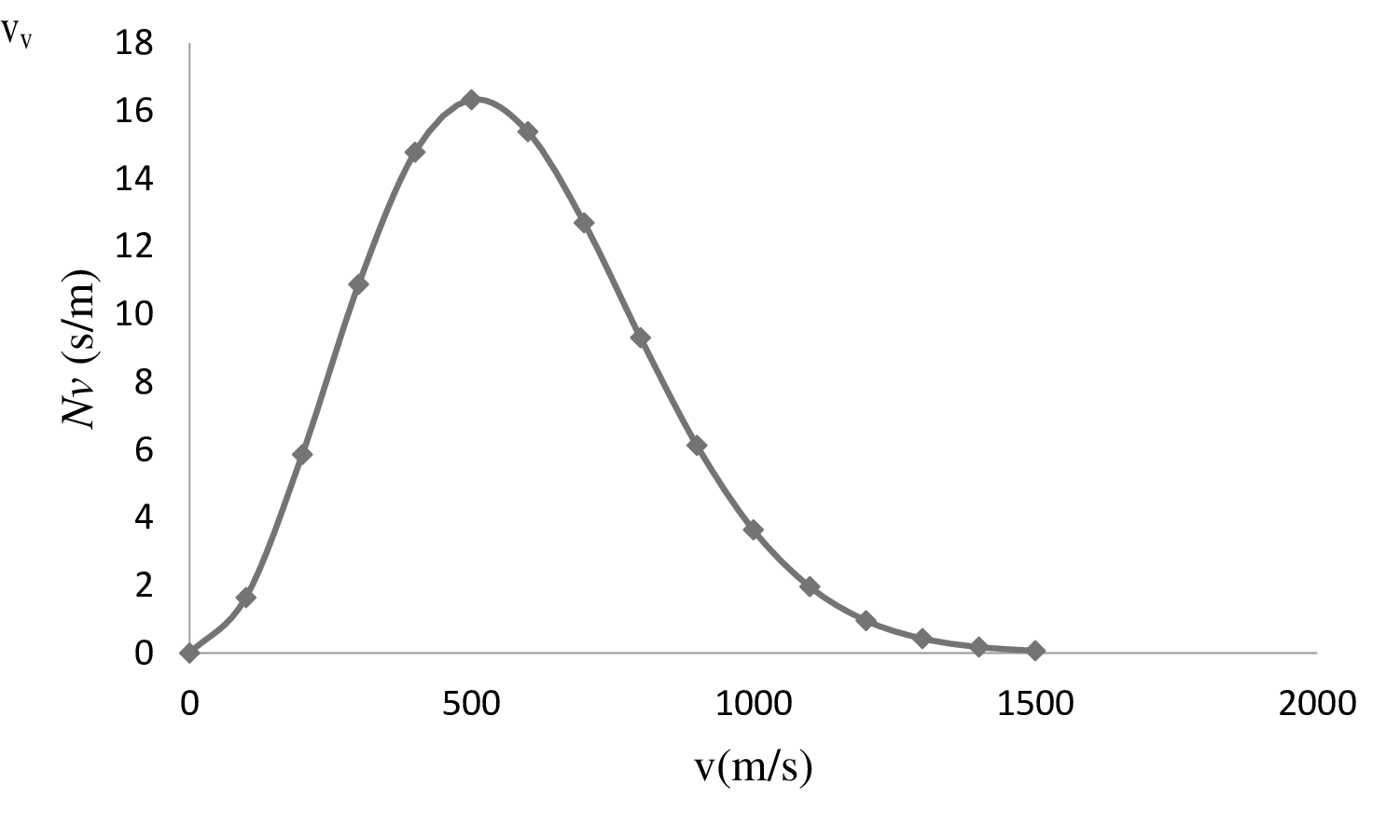
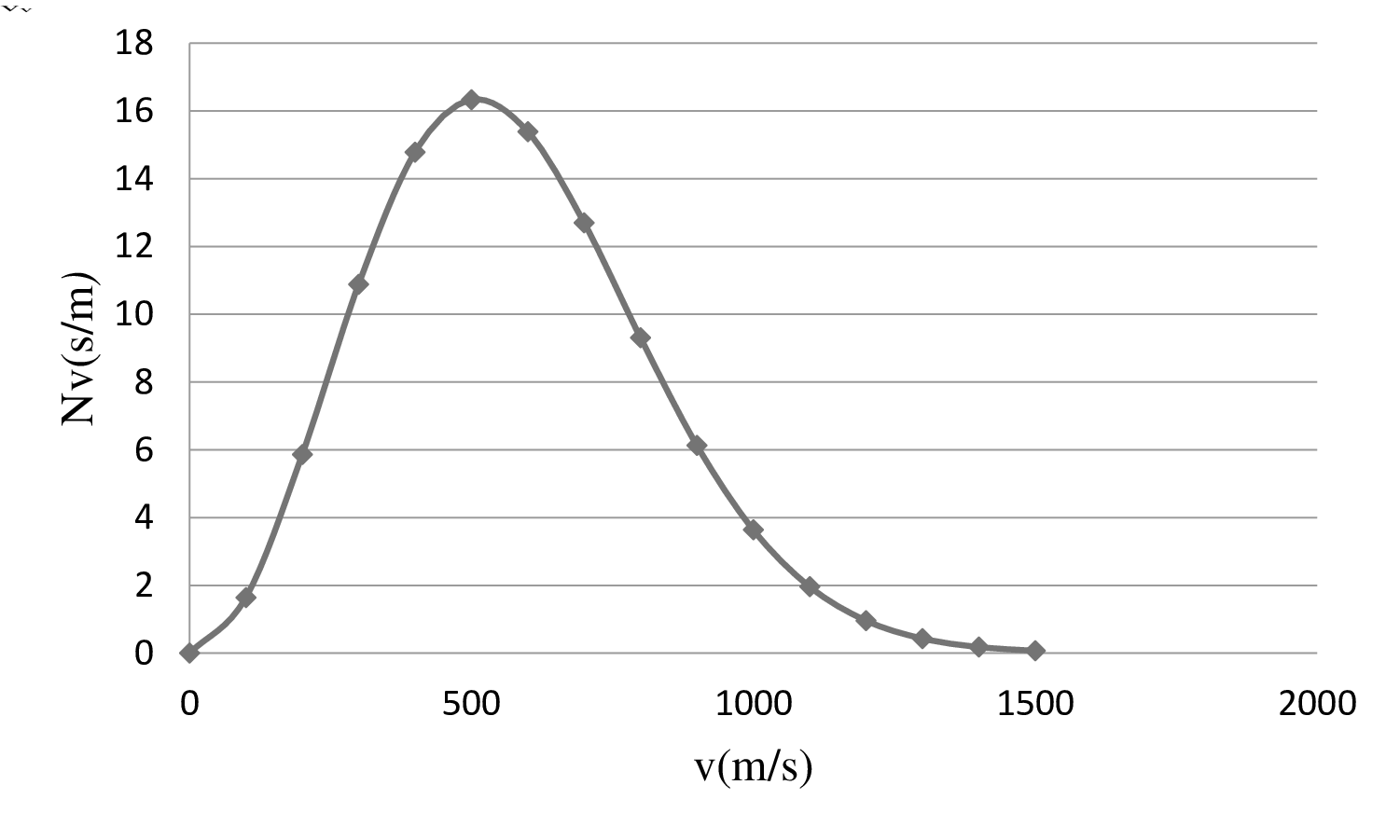
| 0 | 0 |
| 100 | 1.64 |
| 200 | 5.86 |
| 300 | 10.88 |
| 400 | 14.78 |
| 500 | 16.33 |
| 600 | 15.39 |
| 700 | 12.7 |
| 800 | 9.31 |
| 900 | 6.13 |
| 1000 | 3.64 |
| 1100 | 1.961 |
| 1200 | 0.96 |
| 1300 | 0.43 |
| 1400 | 0.18 |
| 1500 | 0.07 |
On the basis of the table, a graph is plotted below;
(b)
The most probable speed from the graph.
Answer to Problem 38AP
The most probable speed is
Explanation of Solution
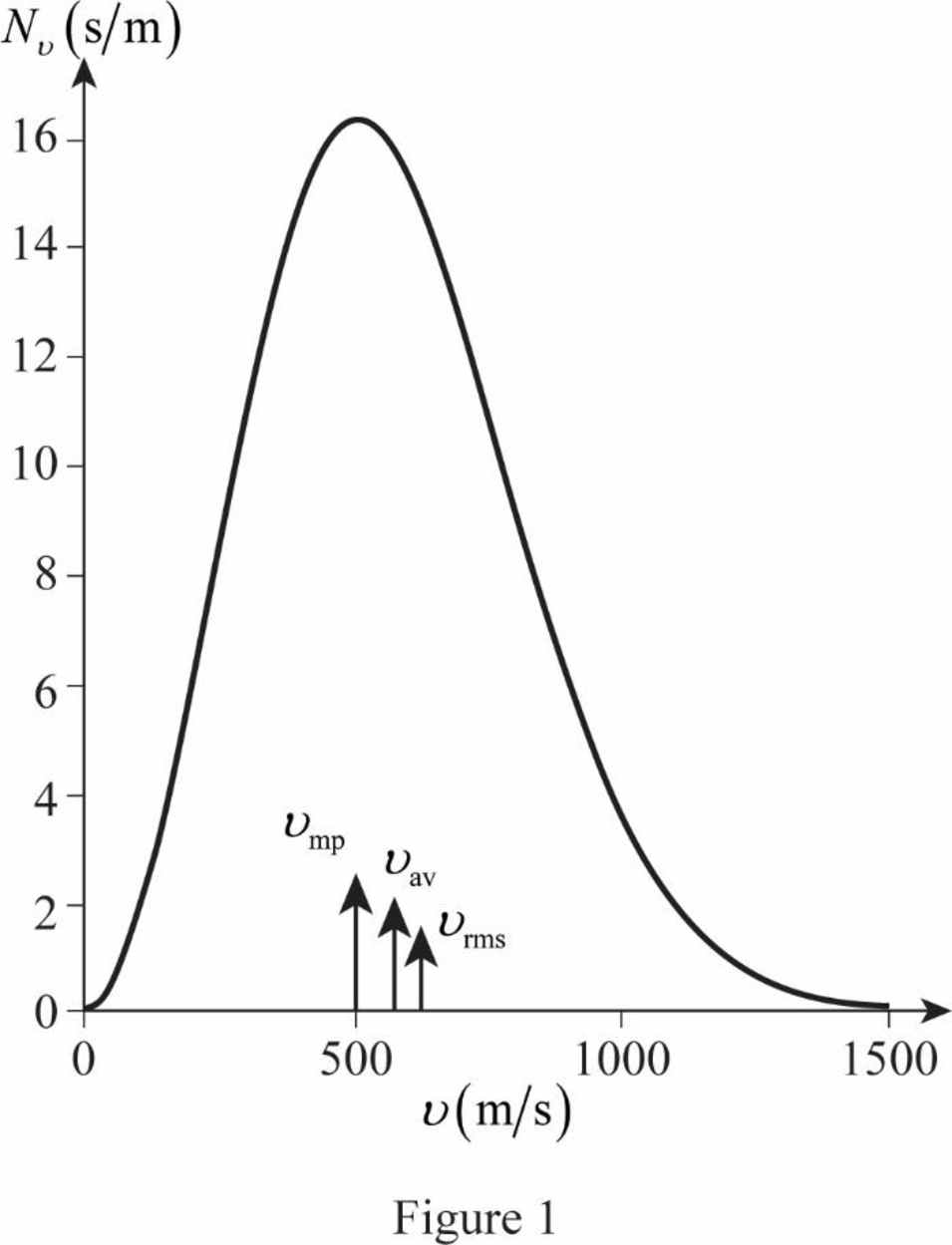
The most probable speed occurs where
Conclusion:
Therefore, the most probable speed is
(c)
The average and rms speeds for the molecules and label these points on the graph.
Answer to Problem 38AP
The average and rms speeds for the molecules is
Explanation of Solution
Write the expression of average velocity.
The mass of the molecules of oxygen
Substitute
The molecular mass of the oxygen molecules in
Substitute
Thus, the average speed is
Write the expression of rms velocity.
Substitute
Substitute
Thus, the rms velocity of the oxygen molecules is
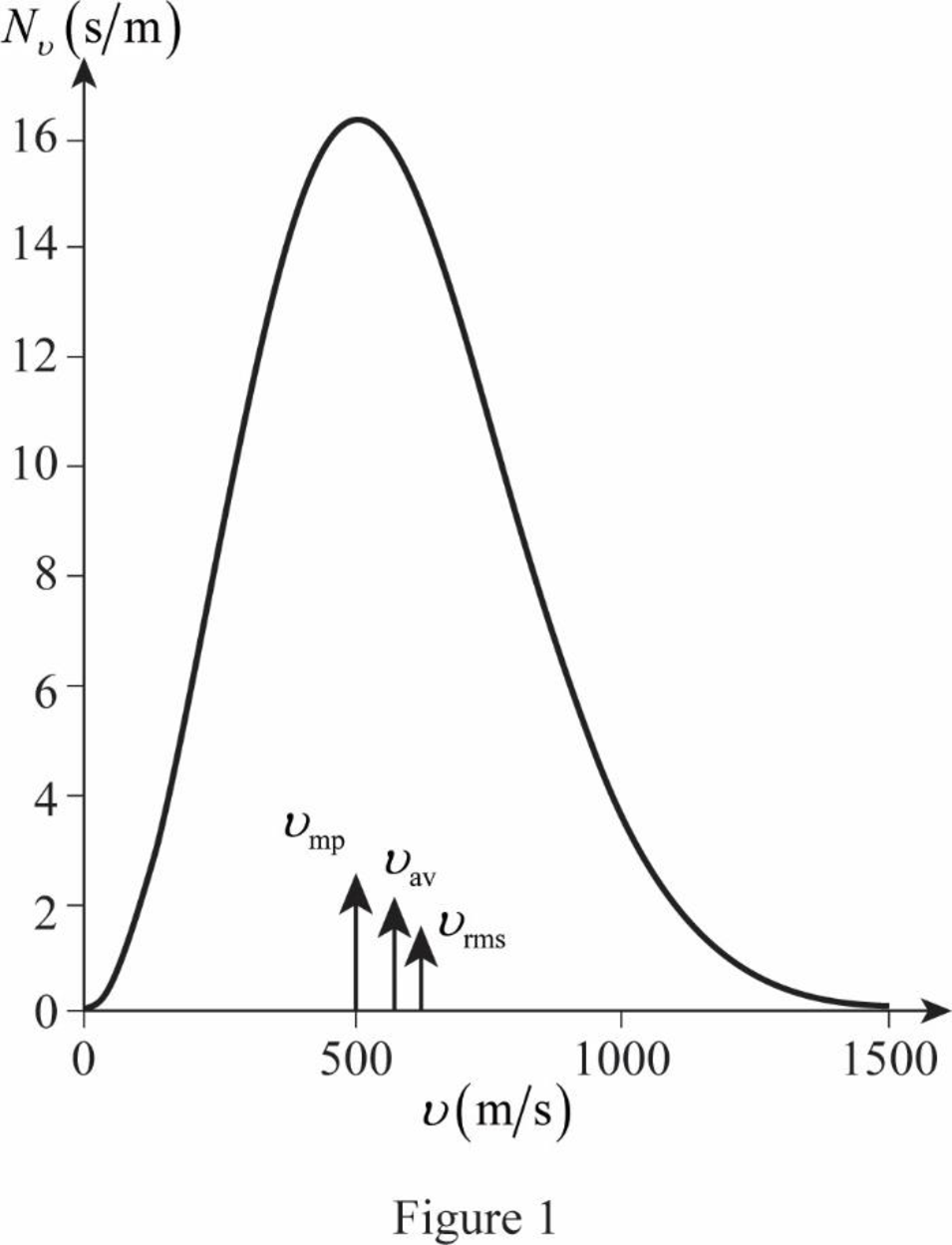
The graph of Maxwell’s curve is shown below;
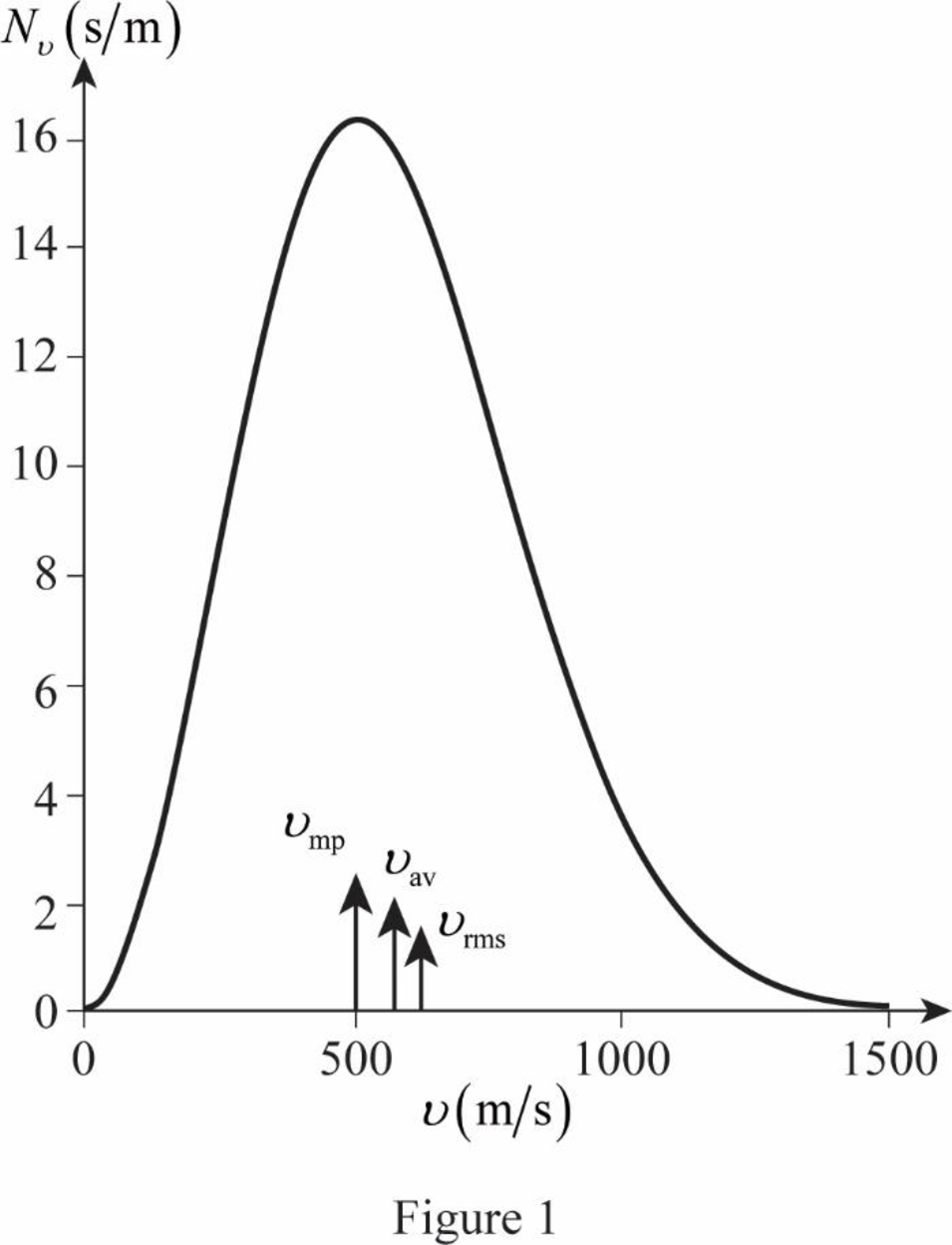
The point
Conclusion:
Therefore, the average and rms speeds for the molecules is
(d)
The fraction of molecules with the speed in the range of
Answer to Problem 38AP
The fraction of molecules with the speed in the range of
Explanation of Solution
The figure given below shows the Maxwell’s curve,
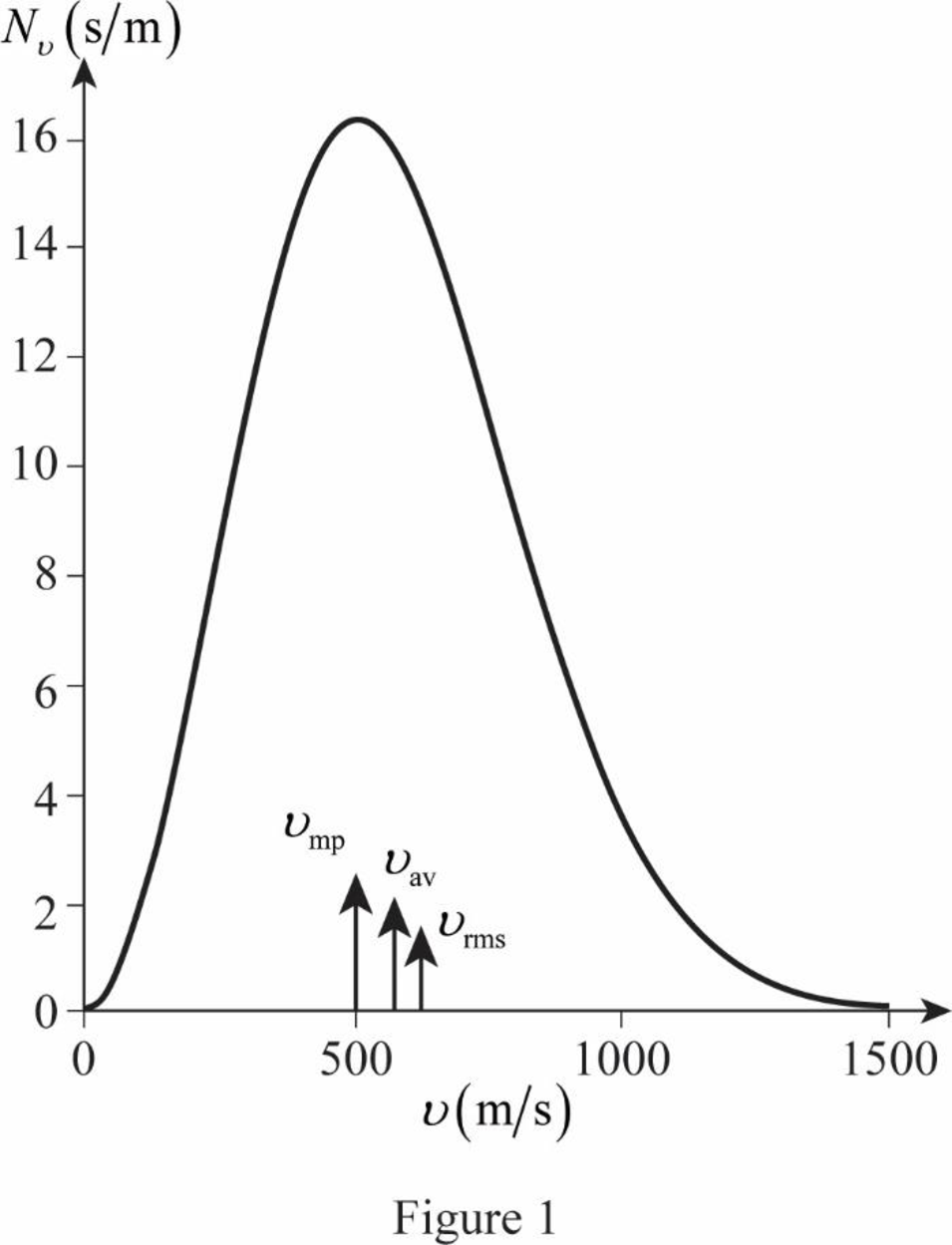
The area under the distribution curve in the range
Conclusion:
Write the area under the curve in the range
Therefore, the fraction of molecules with the speed in the range of
Want to see more full solutions like this?
Chapter 20 Solutions
Bundle: Physics for Scientists and Engineers, Volume 2, Loose-leaf Version, 10th + WebAssign Printed Access Card, Single-Term
- 2. Consider the situation described in problem 1 where light emerges horizontally from ground level. Take k = 0.0020 m' and no = 1.0001 and find at which horizontal distance, x, the ray reaches a height of y = 1.5 m.arrow_forward2-3. Consider the situation of the reflection of a pulse at the interface of two string described in the previous problem. In addition to the net disturbances being equal at the junction, the slope of the net disturbances must also be equal at the junction at all times. Given that p1 = 4.0 g/m, H2 = 9.0 g/m and Aj = 0.50 cm find 2. A, (Answer: -0.10 cm) and 3. Ay. (Answer: 0.40 cm)please I need to show all work step by step problems 2 and 3arrow_forwardFrom number 2 and 3 I just want to show all problems step by step please do not short cut look for formulaarrow_forward
- Look at the answer and please show all work step by steparrow_forward3. As a woman, who's eyes are h = 1.5 m above the ground, looks down the road sees a tree with height H = 9.0 m. Below the tree is what appears to be a reflection of the tree. The observation of this apparent reflection gives the illusion of water on the roadway. This effect is commonly called a mirage. Use the results of questions 1 and 2 and the principle of ray reversibility to analyze the diagram below. Assume that light leaving the top of the tree bends toward the horizontal until it just grazes ground level. After that, the ray bends upward eventually reaching the woman's eyes. The woman interprets this incoming light as if it came from an image of the tree. Determine the size, H', of the image. (Answer 8.8 m) please show all work step by steparrow_forwardNo chatgpt pls will upvotearrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





