
To review:
The change in the appearance of the zones of inhibition of the different antimicrobials if the medication concentrations in the strips were slightly decreased.
Introduction:
MIC is defined as the minimum inhibitory concentration. E-test is a process by which MIC can be determined for an antimicrobial agent. It is determined by reading the number where the growth of the microorganisms intersects the strips. A teardrop-shaped zone of inhibition is noted due to the gradient of concentration of the antimicrobials. A slight decrease in the concentration of antimicrobials will produce a narrower teardrop shaped zone of inhibition as compared to the one given in Figure 20.12.
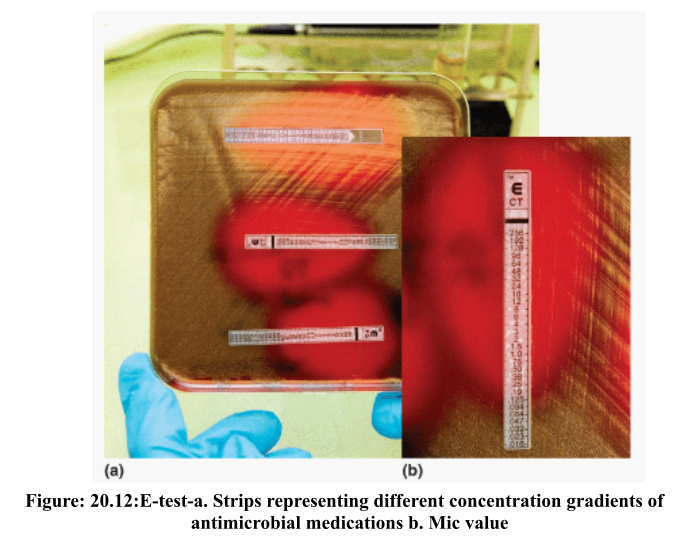
In Figure 20.12, the image of an E-test is given showing the MIC determination. It shows the multiple strips containing a gradient of concentrations of antimicrobials. The MIC is shown by reading the number where the growth intersects the strip.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
NESTER'S MICROBIOLOGY-CONNECT >CUSTOM<
- Molecular Biology Question You are working to characterize a novel protein in mice. Analysis shows that high levels of the primary transcript that codes for this protein are found in tissue from the brain, muscle, liver, and pancreas. However, an antibody that recognizes the C-terminal portion of the protein indicates that the protein is present in brain, muscle, and liver, but not in the pancreas. What is the most likely explanation for this result?arrow_forwardMolecular Biology Explain/discuss how “slow stop” and “quick/fast stop” mutants wereused to identify different protein involved in DNA replication in E. coli.arrow_forwardMolecular Biology Question A gene that codes for a protein was removed from a eukaryotic cell and inserted into a prokaryotic cell. Although the gene was successfully transcribed and translated, it produced a different protein than it produced in the eukaryotic cell. What is the most likely explanation?arrow_forward
- Molecular Biology LIST three characteristics of origins of replicationarrow_forwardMolecular Biology Question Please help. Thank you For E coli DNA polymerase III, give the structure and function of the b-clamp sub-complex. Describe how the structure of this sub-complex is important for it’s function.arrow_forwardMolecular Biology LIST three characteristics of DNA Polymerasesarrow_forward
- Molecular Biology RNA polymerase core enzyme structure contains what subunits? To form holo enzyme, sigma factor is added to core. What is the name of the structure formed? Give the detailed structure of sigma factor and the function of eachdomain. Please help. Thank youarrow_forwardMolecular Biology You have a single bacterial cell whose DNA is labelled with radioactiveC14. After 5 rounds of cell division, how may cells will contain radioactive DNA? Please help. Thank youarrow_forward1. Explain the structure and properties of atoms and chemical bonds (especially how they relate to DNA and proteins). Also add some pictures.arrow_forward
- 1. In the Sentinel Cell DNA integrity is preserved through nanoscopic helicase-coordinated repair, while lipids in the membrane are fortified to resist environmental mutagens. also provide pictures for this question.arrow_forwardExplain the structure and properties of atoms and chemical bonds (especially how they relate to DNA and proteins). Also add some pictures.arrow_forwardIn the Sentinel Cell DNA integrity is preserved through nanoscopic helicase-coordinated repair, while lipids in the membrane are fortified to resist environmental mutagens. also provide pictures for this question.arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage




