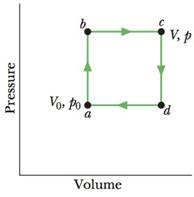
Problem 1Q: Point i in Fig. 20-19 represents the initial state of an ideal gas at temperature T. Taking... Problem 2Q: In lour experiments, blocks A and B, starting ill different initial temperatures, were brought... Problem 3Q: A gas, confined to an insulated cylinder, is compressed adiabatically to half its volume. Does the... Problem 4Q: An ideal monatomic gas at initial temperature T0 in kelvins expands from initial volume V0 to volume... Problem 5Q: In four experiments, 2.5 mol of hydrogen gas under-goes reversible isothermal expansions, starting... Problem 6Q: A box contains 100 atoms in a configuration that has 50 atoms in each half of the box. Suppose that... Problem 7Q: Does the entropy per cycle increase, decrease, or remain the same for a a Carnot engine, b a real... Problem 8Q: Three Carnot engines operate between temperature limits of a 400 and 500 K, b 500 and 600 K, and c... Problem 9Q: An inventor claims to have invented four engines, each of which operates between... Problem 10Q: Does the entropy per cycle increase, decrease, or remain the same for a a Carnot refrigerator, b a... Problem 1P: SSM Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume V1 to... Problem 2P Problem 3P: ILW A 2.50 mol sample of an ideal gas expands reversibly and isothermally at 360 K until its volume... Problem 4P Problem 5P: ILW Find a the energy absorbed as heat and b the change in entropy of a 2.00 kg block of copper... Problem 6P: a What is the entropy change of a 12.0 g ice cube that melts completely in a bucket of water whose... Problem 7P: ILW A 50.0 g block of copper whose temperature is 400 K is placed in an insulating box with a 100 g... Problem 8P: At very low temperatures, the molar specific heat Cv of many solids is approximately Cv = AT3, where... Problem 9P: A 10 g ice cube at 10oC is placed in a lake whose temperature is 15C. Calculate the change in... Problem 10P: A 364 g block is put in contact with a thermal reservoir. The block is initially at a lower... Problem 11P: SSM WWW In an experiment, 200 g of aluminum with a specific heat of 900 J/kg K at 100C is mixed... Problem 12P: A gas sample undergoes a reversible isothermal expansion. Figure 20-23 gives the change S in entropy... Problem 13P: In the irreversible process of Fig. 20-5, let the initial temperatures of the identical blocks L and... Problem 14P Problem 15P: A mixture of 1773 g of water and 227 g of ice is in an initial equilibrium state at 0.000C. The... Problem 16P: GO An 8.0 g ice cube at -10C is put into a Thermos flask containing 100 cm3 of water at 20C. By how... Problem 17P Problem 18P: GO A 2.0 mol sample of an ideal monatomic gas undergoes the reversible process shown in Fig. 20-26.... Problem 19P: Suppose 1.00 mol of a monatomic ideal gas is taken from initial pressure p1 and volume V1 through... Problem 20P: Expand 1.00 mol of an monatomic gas initially at 5.00 kPa and 600 K from initial volume Vi = 1.00 m3... Problem 21P: GO Energy can be removed from water as heat at and even below the normal freezing point 0.0C at... Problem 22P: GO An insulated Thermos contains 130 g of water at 80.0C. You put in a 12.0 g ice cube at 0C to form... Problem 23P: A Carnot engine whose low-temperature reservoir is at 17C has an efficiency of 40. By how much... Problem 24P: A Carnot engine absorbs 52 kJ as heat and exhausts 36 kJ as heat in each cycle. Calculate a the... Problem 25P: A Carnot engine has an efficiency of 22.0. It operates between constant-temperature reservoirs... Problem 26P: In a hypothetical nuclear fusion reactor, the fuel is deuterium gas at a temperature of 7 108 K. If... Problem 27P: SSM WWW A Carnot engine operates between 235C and 115C absorbing 630 104 J per cycle at the higher... Problem 28P: In the first stage of a two-stage Carnot engine, energy is absorbed as heat Q1 at temperature T1... Problem 29P: GO Figure 20-27 shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is taken.... Problem 30P: A 500 W Carnot engine operates between constant-temperature reservoirs at 100C and 60.0C. What is... Problem 31P: The efficiency of a particular car engine is 25 when the engine does 8.2 kJ of work per cycle.... Problem 32P: GO A Carnot engine is set up to produce a certain work W per cycle. In each cycle, energy in the... Problem 33P: SSM ILW Figure 20-29 shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is... Problem 34P: GO An ideal gas 1.0 mol is the working substance in an engine that operates on the cycle shown in... Problem 35P: The cycle in Fig. 20-31 represents the operation of a gasoline internal combustion engine. Volume V3... Problem 36P: How much work must be done by a Carnot refrigerator to transfer 1.0 J as heat a from a reservoir at... Problem 37P: SSM A heat pump is used to heal a building, The external temperature is less than the Internal... Problem 38P: The electric motor of a heat pump transfers energy as heat from the outdoors, which is at 5.0C, to a... Problem 39P: SSM A Carnot air conditioner lakes energy from the thermal energy of a room at 70F and transfers it... Problem 40P: To make ice, a freezer that is a reverse Carnot engine extracts 42 kJ as heat at 15C during each... Problem 41P: ILW An air conditioner operating between 93F and 70F is rated at 4000 Btu/h cooling capacity. Its... Problem 42P: The motor in a refrigerator has a power of 200 W. If the freezing compartment is at 270 K and the... Problem 43P: GO Figure 20-32 represents a Carnot engine that works between temperatures T1 = 400 K and T2= 150 K... Problem 44P: a During each cycle, a Carnot engine absorbs 750 J as heat from a high-temperature reservoir at 360... Problem 45P Problem 46P: A box contains N identical gas molecules equally divided between its two halves. For N = 50, what... Problem 47P: SSM WWW A box contains N gas molecules, Consider the box to be divided into three equal parts a By... Problem 48P: Four particles are in the insulated box of Fig. 20-17. What are a the least multiplicity, b the... Problem 49P: A cylindrical copper rod of length 1.50 m and radius 2.00 cm a insulted to prevent heat loss through... Problem 50P: Suppose 0.550 mol of an ideal gas is isothermally and reversibly expanded in the four situations... Problem 51P Problem 52P: Suppose 1.0 mol of a monatomic ideal gas initially at 10 L and 300 K is heated at constant volume to... Problem 53P: GO Suppose that a deep shaft were drilled in Earths crust near one of the poles, where the surface... Problem 54P: What is the entropy change for 3.20 mol of an ideal monatomic gas undergoing a reversible Increase... Problem 55P: A 600 g lump of copper at 80.0C is placed in 70.0 g of water at 10.0C in an insulated container. See... Problem 56P: Figure 20-33 gives the force magnitude F versus stretch distance x for a rubber band, with the scale... Problem 57P: The temperature of 1.00 mol of a monatomic ideal gas is raised reversibly from 300 K to 400 K, with... Problem 58P: Repeat Problem 57, with the pressure now kept constant. 57 The temperature of 1.00 mol of a... Problem 59P Problem 60P: A three-step cycle is undergone by 3.4 mol of an ideal diatomic gas: 1 the temperature of the gas is... Problem 61P: An inventor has built an engine X and claims that its efficiency X is greater than the efficiency ... Problem 62P: Suppose 2.00 mol of a diatomic gas is taken reversibly around the cycle shown in the T-S diagram of... Problem 63P: A three-step cycle is undergone reversibly by 4.00 mol of an ideal gas: 1 an adiabatic expansion... Problem 64P: a A Carnot engine operates between a hot reservoir at 320 K and a cold one at 260 K. If the engine... Problem 65P: A 2.00 mol diatomic gas initially at 300 K undergoes this cycle: It is 1 heated at constant volume... Problem 66P: An ideal refrigerator does 150 J of work to remove 560 J as heat from its cold compartment, a What... Problem 67P: Suppose that 260 J is conducted from a constant-temperature reservoir at 400 K to one at a 100 K, b... Problem 68P: An apparatus that liquefies helium is in a room maintained at 300 K. If the helium in the apparatus... Problem 69P: GO A brass rod is in thermal contact with a constant-temperature reservoir at 130C at one end and a... Problem 70P: A 45.0 g block of tungsten at 30.0C and a 25.0 g block of silver at 120C are placed together in an... Problem 71P Problem 72P: Calculate the efficiency of a fossil-fuel power plant that consumes 380 metric tons of coal each... Problem 73P: SSM A Carnot refrigerator extracts 35.0 kJ as heat during each cycle, operating with a coefficient... Problem 74P: A Carnot engine whose high-temperature reservoir is at 400 K has an efficiency of 30.0. By how much... Problem 75P: SSM System A of three particles and system B of five particles are in insulated boxes like that in... Problem 76P: Figure 20-36 shows a Carnot cycle on a T-S diagram, with a scale set by Ss = 0.60 J/K. For a full... Problem 77P: Find the relation between the efficiency of a reversible ideal heat engine and the coefficient of... Problem 78P: A Carnot engine has a power of 500 W. It operates between heat reservoirs at 100C and 60.0C.... Problem 79P: In a real refrigerator, the low-temperature coils are at 13C, and the compressed gas in the... format_list_bulleted



 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning




