
(a)
Interpretation:
6-phosphoglucono-⏹-lactone should be identified.
Concept introduction:
6-phosphoglucono-⏹-lactone is formed during the pentose phosphate pathway. In the first step of pentose phosphate pathway, dehydrogenation of glucose-6-phosphate at C-1 takes produce 6-phosphoglucono-⏹-lactone.
Answer to Problem 28P
The compound C in the reaction is 6-phosphoglucono-⏹-lactone.
Explanation of Solution
6-phosphoglucono-⏹-lactone is an intramolecular ester formed by the reaction of C-1 carboxyl group and C-5 hydroxyl group. It is formed by dehydrogenation of C-1 carbon of Glucose-6-phosphate. Hydroxyl group at C-1 of glucose-6-phosphate is converted to carbonyl group. Therefore, the structure of 6-phosphoglucono-⏹-lactone is (C).
(b)
Interpretation:
The reactions producing NADPH should be determined.
Concept introduction:
Two molecules of NADPH are produced duringthe oxidative phase of pentose phosphate pathway.
Answer to Problem 28P
The reactions B and F produce NADPH.
Explanation of Solution
First NADPH is produced when the C-1 in glucose-6-phosphate is dehydrogenated into 6-phosphoglucono-⏹-lactone by glucose-6-phosphate dehydrogenase. This 6-phosphoglucono-⏹-lactone is hydrolyzed by a lactonase resulting 6-phosphogluconate. This 6C sugar acid is then decarboxylated by 6-phosphogluconate dehydrogenase into ribulose-5-phosphate. In this step also NADP+ acts as the electron acceptor and produce NADPH.
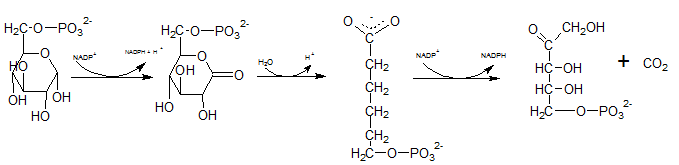
Therefore,reactions B and F produces NADPH.
(c)
Interpretation:
Ribulose-5-phosphate should be identified.
Concept introduction:
Ribulose-5-phosphate is the productof pentose phosphate pathway.
Answer to Problem 28P
The compound G in the reaction is Ribulose-5-phosphate.
Explanation of Solution
As the first step of oxidative phase of pentose phosphate pathway, C-1 of glucose-6-phosphate is dehydrogenated into 6-phosphoglucono-⏹-lactone by glucose-6-phosphate dehydrogenase. Then this 6-phosphoglucono-⏹-lactone is hydrolyzed by a lactonase resulting 6-phosphogluconate. This 6 C sugar acid is then decarboxylated by 6-phosphogluconate dehydrogenase into ribulose-5-phosphate.
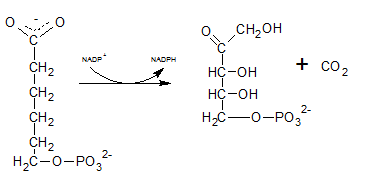
Therefore, Gis Ribulose-5-phosphate.
(d)
Interpretation:
The CO2generating reaction should be determined.
Concept introduction:
The decarboxylation reactions generate CO2 as a by-product.
Answer to Problem 28P
The reactions F produce CO2.
Explanation of Solution
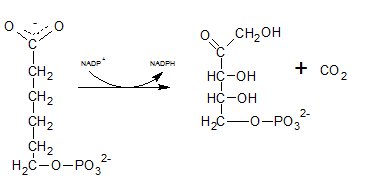
The six C sugar acid, 6-phosphogluconate formed during pentose phosphate pathway is oxidatively decarboxylated by 6-phosphogluconate dehydrogenase into ribulose-5-phosphate. The final product is a five-carbon sugar, and release CO2.
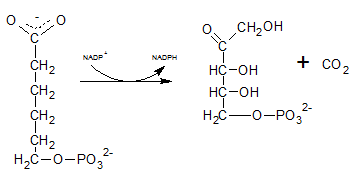
So. the reaction F produce CO2.
(e)
Interpretation:
6-phosphogluconate should be identified.
Concept introduction:
6-phosphogluconate is a 6C sugar acid which forms during pentose phosphate pathway.
Answer to Problem 28P
The compound E in the reaction is 6-phosphogluconate.
Explanation of Solution
In the first step of the oxidative phase of pentose phosphate pathway, C-1 of glucose-6-phosphate is dehydrogenated into 6-phosphoglucono-⏹-lactone by glucose-6-phosphate dehydrogenase which is hydrolyzed by a lactonase resulting 6-phosphogluconate.
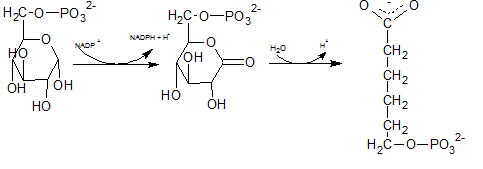
The end product of above reaction is 6-phosphogluconate. Thus the compound E in the reaction is 6-phosphogluconate.
(f)
Interpretation:
The reaction that is catalyzed by phosphopentose isomerase should be determined.
Concept introduction:
Phosphopentose isomerase is an enzyme which involves in isomerization reaction.
Answer to Problem 28P
The reactions H uses Phosphopentose isomerase enzyme.
Explanation of Solution
Ribulose-5-phosphate is isomerized to ribose-5-phosphate by phosphopentose isomerase. The enzyme, phosphopentose isomerase catalyze the conversion of a ketose sugar (Ribulose-5-phosphate ) to an aldose sugar (ribose-5-phosphate).
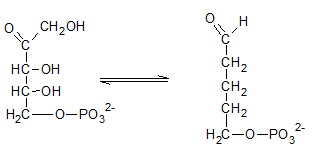
Therefore, the reaction H needs the enzyme phosphopentose isomerase.
(g)
Interpretation:
Ribose-5-phosphate should be identified.
Concept introduction:
Ribose-5-phosphate is the end product of the oxidative phase of pentose phosphate pathway.
Answer to Problem 28P
The compound I in the reaction is Ribose-5-phosphate.
Explanation of Solution
The ribulose-5-phosphate is obtained in pentose phosphate pathway when the ribose-5-phosphate is isomerized by phosphopentose isomerase.
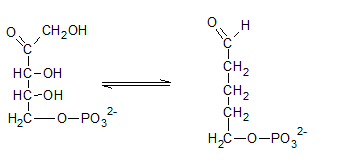
Therefore, the compound I in the reaction is Ribose-5-phosphate.
(h)
Interpretation:
Reaction catalyzed by lactonase should be determined.
Concept introduction:
Lactonases catalyzes the hydrolysis of ester bonds.
Answer to Problem 28P
The reactions D uses the enzymeLactonases.
Explanation of Solution
The degydrogenated product of glucose-6-phospahte is 6-phosphoglucono-⏹-lactone. This is a 6 membered ring structure and have an ester bond between C-1 carbonyl carbon and C-5 hydroxyl Oxygen. This bond is hydrolyzed by lactonase and to produce 6-phosphogluconate. The reaction is indicated by letter D.
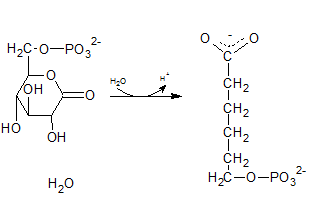
(i)
Interpretation:
Glucose-6-phosphate should be identified.
Concept introduction:
The pentose phosphate pathway is initiated by the oxidation of glucose-6-phosphate.
Answer to Problem 28P
The compound A in the reaction is glucose-6-phosphate.
Explanation of Solution
Glucose-6-phosphate is a 6-carbon sugar and have a ring structure where the hydroxyl group at C-6 is phosphorylated.
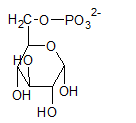
The compound A in the reaction is glucose-6-phosphate.
(j)
Interpretation:
The reaction catalyzed by 6-phosphogluconate dehydrogenase should be determined.
Concept introduction:
Dehydrogenases are the enzymes which catalyzes the removal of hydrogen molecules with the help of coenzymes NAD and FAD.
Answer to Problem 28P
The reactions F uses the enzymedehydrogenases.
Explanation of Solution
6-phosphogluconate is oxidatively decarboxylated by 6-phosphogluconate dehydrogenase into ribulose-5-phosphate which is indicated by reaction F.
(k)
Interpretation:
Reaction that is catalyzed by glucose-6-phosphate dehydrogenase should be determined.
Concept introduction:
In the first reaction of pentose phosphate pathway glucose-6-phosphate is dehydrogenated to produce phosphoglucono-⏹-lactone.
Answer to Problem 28P
The reactions B uses the enzymeglucose-6-phosphate dehydrogenases.
Explanation of Solution
In the first step of pentose phosphate pathway, Glucose-6-phosphate is dehydrogenated at C-1 by glucose-6-phosphate dehydrogenase enzyme into 6-phosphoglucono-⏹-lactone.
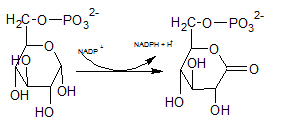
So, the reactions B uses the enzymeglucose-6-phosphate dehydrogenases.
Want to see more full solutions like this?
Chapter 20 Solutions
BIOCHEMISTRY-ACHIEVE (1 TERM)
- You’ve isolated a protein and determined that the Native molecular weight of the holoenzyme is 160 kD using size exclusion chromatography. Analysis of this protein using SDS-PAGE revealed 2 bands, one at 100 kD and one at 30 kD. The enzyme was found to be 0.829% NAD (by weight). What further can be said regarding the structure of the polypeptide?arrow_forwardWhat is the formation of glycosylated hemoglobin (the basis for the HbA1c test)? Can you describe it?arrow_forwardPlease analze the gel electrophoresis column of the VRK1 kinase (MW: 39.71 kDa). Also use a ruler to measure the length of the column in centimeters and calculate the MW of each band observed. Lane 1: buffer Lane 2 : Ladder Lane 3: Lysate Lane 4: Flowthrough Lane 5: Wash Lanes 6-8: E1, E2, E3 Lane 9: Dialyzed VRK1 Lane 10: LDHarrow_forward
- Do sensory neurons express ACE2 or only neurolipin-1 receptors for COVID19 virus particle binding?arrow_forwardExplain the process of CNS infiltration of COVID19 through sensory neurons from beginning to end, including processes like endocytosis, the different receptors/proteins that are involved, how they are transported and released, etc.,arrow_forwardH2C CH2 HC-COOO CH2 ܘHO-C-13c-O isocitrate C-S-COA H213c CH2 C-OO 13C-S-COA CH2 C-00 the label will not be present in succinyl CoA C-S-COA succinyl-CoAarrow_forward
- A culture of kidneys cells contains all intermediates of the citric acid cycle. It is treated with an irreversible inhibitor of malate dehydrogenase, and then infused withglucose. Fill in the following list to account for the number of energy molecules that are formed from that one molecule of glucose in this situation. (NTP = nucleotidetriphosphate, e.g., ATP or GTP)Net number of NTP:Net number of NADH:Net number of FADH2:arrow_forward16. Which one of the compounds below is the final product of the reaction sequence shown here? OH A B NaOH Zn/Hg aldol condensation heat aq. HCI acetone C 0 D Earrow_forward2. Which one of the following alkenes undergoes the least exothermic hydrogenation upon treatment with H₂/Pd? A B C D Earrow_forward
- 6. What is the IUPAC name of the following compound? A) (Z)-3,5,6-trimethyl-3,5-heptadiene B) (E)-2,3,5-trimethyl-1,4-heptadiene C) (E)-5-ethyl-2,3-dimethyl-1,5-hexadiene D) (Z)-5-ethyl-2,3-dimethyl-1,5-hexadiene E) (Z)-2,3,5-trimethyl-1,4-heptadienearrow_forwardConsider the reaction shown. CH2OH Ex. CH2 -OH CH2- Dihydroxyacetone phosphate glyceraldehyde 3-phosphate The standard free-energy change (AG) for this reaction is 7.53 kJ mol-¹. Calculate the free-energy change (AG) for this reaction at 298 K when [dihydroxyacetone phosphate] = 0.100 M and [glyceraldehyde 3-phosphate] = 0.00300 M. AG= kJ mol-1arrow_forwardIf the pH of gastric juice is 1.6, what is the amount of energy (AG) required for the transport of hydrogen ions from a cell (internal pH of 7.4) into the stomach lumen? Assume that the membrane potential across this membrane is -70.0 mV and the temperature is 37 °C. AG= kJ mol-1arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning





