
(a)
Interpretation:
A synthesis of the given compound by using acetyl chloride is to be determined.
Concept introduction:
Sodium benzoate is the salt of conjugate base or sodium salt of benzoic acid. When acyl halide reacts with sodium benzoate, it gives the ester. Sodium benzoate acts as the nucleophile. Nucleophile is the negatively charged ion attacks the carbonyl carbon and expels the good leaving group.
Answer to Problem 20.65P
The way of synthesis of the given compound by using acetyl chloride is as follows:
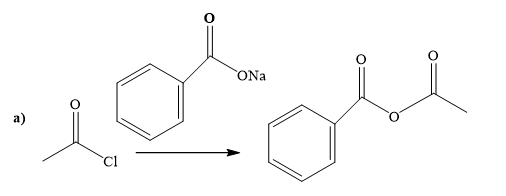
Explanation of Solution
The way of synthesis of the given compound by using acetyl chloride is as follows:

Sodium benzoate is a sodium salt of benzoic acid. When acyl halide reacts with sodium benzoate, it gives the ester. Sodium benzoate, as s nucleophile, is used to undergo nucleophilic addition- elimination reaction with acid chloride. Benzoate’s negatively charged ion attacks the carbonyl carbon of acetyl chloride and expels the good leaving group chlorine.
From the structure of the given reactant and product, structure of the reagent is determined.
(b)
Interpretation:
A synthesis of the given compound by using the acetyl chloride is to be determined.
Concept introduction:
Sodium phenoxide is produced by sodium hydroxide and Phenol. Ester can be prepared by treating acetyl chloride with phenoxide. Sodium phenoxide is a moderately strong base.
Answer to Problem 20.65P
The way of synthesis of the given compound by using acetyl chloride is as follows:
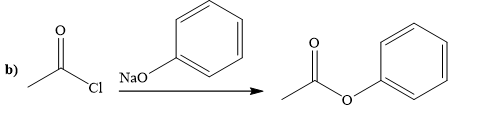
Explanation of Solution
The way of synthesis of the given compound by using acetyl chloride is as follows:

Ester can be prepared by treating acetyl chloride with sodium phenoxide. Sodium phenoxide, as a nucleophile, is used to undergo nucleophilic addition- elimination reaction with acid chloride. Phenoxide, the negatively charged ion attacks the carbonyl carbon of acetyl chloride and expels the good leaving group chlorine and forms ester.
From the structure of given reactant and product, structure of reagent is determined.
(c)
Interpretation:
A synthesis of the given compound by using acetyl chloride is to be determined.
Concept introduction:
It converts the acid chloride or anhydrides to primary alcohol. It’s also used for reduction of carbonyl compound to corresponding alcohol, and after removing the hydrogen from alcohol, it gives alkoxide. Alkoxide ion attacks the carbonyl carbon of the acid chloride and expels the good leaving group and forms the ester as product.
Answer to Problem 20.65P
The way of synthesis of the given compound by using acetyl chloride is as follows:
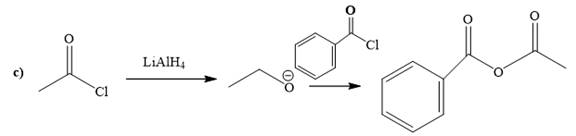
Explanation of Solution
The way of synthesis of the given compound by using acetyl chloride is as follows:

Acetyl halide is converted to alkoxide by treating it with
From the structure of the given reactant and product, structure of reagent is determined.
(d)
Interpretation:
A synthesis of the given compound by using acetyl chloride is to be determined.
Concept introduction:
It is also used for reduction of amides to corresponding
Answer to Problem 20.65P
The way of synthesis of given compound by using acetyl chloride is as follows:
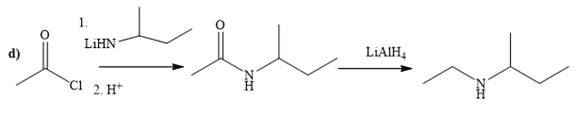
Explanation of Solution
The way of synthesis of given compound by using acetyl chloride is as follows:

In the first step, acid chloride converts to amide in the presence of organolithium amine reagent. Amide is reduced with
From the structure of given reactant and product, structure of reagent is determined.
(e)
Interpretation:
A synthesis of given compound by using the acetyl chloride is to be determined.
Concept introduction:
It converts the acid chloride or anhydrides to primary alcohol. It’s also used for reduction of carbonyl a compound to corresponding alcohol. And after remove the hydrogen from alcohol to get alkoxide. Alkoxide ion attacks the carbonyl carbon of the acid chloride and expels the good leaving group and form the ester as product. Alcohol is reacting with strong acid at required temperature to get the ester.
Answer to Problem 20.65P
The way of synthesis of given compound by using acetyl chloride is as follows:
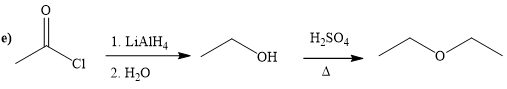
Explanation of Solution
The way of synthesis of given compound by using acetyl chloride is as follows:

In the first step, acetyl chloride is converted to
From the structure of given reactant and product, structure of reagent is determined.
(f)
Interpretation:
A synthesis of given compound by using acetyl chloride is to be determined.
Concept introduction:
The reagent
Answer to Problem 20.65P
The way of synthesis of given compound by using acetyl chloride is as follows:
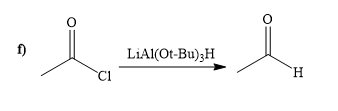
Explanation of Solution
The way of synthesis of given compound by using acetyl chloride is as follows:

When acyl chloride react with
From the structure of given reactant and product, structure of reagent is determined.
(g)
Interpretation:
A synthesis of given compound by using acetyl chloride is to be determined.
Concept introduction:
Acid chloride reacts with Gilmann reagent to give the
Answer to Problem 20.65P
The way of synthesis of given compound by using acetyl chloride is as follows:
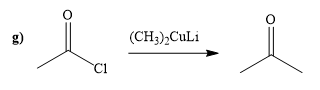
Explanation of Solution
The way of synthesis of given compound by using acetyl chloride is as follows:
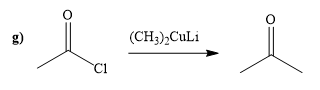
Acetyl chloride is converted to ketone in the presence lithium dimethylcuprate. Ketone does not undergo nucleophilic attack by Gilmann reagent as the reagent is a soft nucleophile.
From the structure of given reactant and product, structure of reagent is determined.
(h)
Interpretation:
A synthesis of given compound by using the acetyl chloride is to be determined.
Concept introduction:
Acid chloride is converted to ester in presence of sodium alkoxide. Alkoxide ion attacks the carbonyl carbon of acid chloride and expels the good leaving group forming ester as the product.
Answer to Problem 20.65P
The way of synthesis of given compound by using acetyl chloride is as follows:
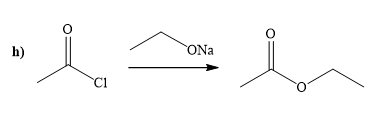
Explanation of Solution
The way of synthesis of given compound by using acetyl chloride is as follows:

Acetyl chloride is converted to ester in presence of sodium ethoxide. Ethoxide ion attacks the carbonyl carbon of acid chloride and expels the good leaving group, forming the ester as a product.
From the structure of given reactant and product, structure of reagent is determined.
Want to see more full solutions like this?
Chapter 20 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- 6. The equilibrium constant for the reaction 2 HBr (g) → H2(g) + Br2(g) Can be expressed by the empirical formula 11790 K In K-6.375 + 0.6415 In(T K-¹) - T Use this formula to determine A,H as a function of temperature. Calculate A,-H at 25 °C and at 100 °C.arrow_forward3. Nitrosyl chloride, NOCI, decomposes according to 2 NOCI (g) → 2 NO(g) + Cl2(g) Assuming that we start with no moles of NOCl (g) and no NO(g) or Cl2(g), derive an expression for Kp in terms of the equilibrium value of the extent of reaction, Seq, and the pressure, P. Given that K₂ = 2.00 × 10-4, calculate Seq/ of 29/no when P = 0.080 bar. What is the new value по ƒª/ at equilibrium when P = 0.160 bar? Is this result in accord with Le Châtelier's Principle?arrow_forwardConsider the following chemical equilibrium: 2SO2(g) + O2(g) = 2SO3(g) • Write the equilibrium constant expression for this reaction. Now compare it to the equilibrium constant expression for the related reaction: • . 1 SO2(g) + O2(g) = SO3(g) 2 How do these two equilibrium expressions differ? What important principle about the dependence of equilibrium constants on the stoichiometry of a reaction can you learn from this comparison?arrow_forward
- Given Kp for 2 reactions. Find the Kp for the following reaction: BrCl(g)+ 1/2 I2(g) ->IBr(g) + 1/2 Cl2(g)arrow_forwardFor a certain gas-phase reaction at constant pressure, the equilibrium constant Kp is observed to double when the temperature increases from 300 K to 400 K. Calculate the enthalpy change of the reaction, Ah, using this information.arrow_forwardHydrogen bonding in water plays a key role in its physical properties. Assume that the energy required to break a hydrogen bond is approximately 8 kJ/mol. Consider a simplified two-state model where a "formed" hydrogen bond is in the ground state and a "broken" bond is in the excited state. Using this model: • Calculate the fraction of broken hydrogen bonds at T = 300 K, and also at T = 273 K and T = 373 K. • At what temperature would approximately 50% of the hydrogen bonds be broken? • What does your result imply about the accuracy or limitations of the two-state model in describing hydrogen bonding in water? Finally, applying your understanding: • Would you expect it to be easier or harder to vaporize water at higher temperatures? Why? If you were to hang wet laundry outside, would it dry more quickly on a warm summer day or on a cold winter day, assuming humidity is constant?arrow_forward
- (3 pts) Use the Kapustinskii equation to calculate the lattice enthalpy for MgBr2 anddiscuss any differences between this result and that from #4.arrow_forward(3 pts) Silver metal adopts a fcc unit cell structure and has an atomic radius of 144 pm. Fromthis information, calculate the density of silver. Show all work.arrow_forward4. (3 pts) From the information below, determine the lattice enthalpy for MgBr2. Show all work. AH/(kJ mol-¹) Sublimation of Mg(s) +148 lonization of Mg(g) +2187 to Mg2+(g) Vaporization of Br₂(1) +31 Dissociation of Br,(g) +193 Electron gain by Br(g) -331 Formation of MgBr₂(s) -524arrow_forward
- 1. (4 pts-2 pts each part) Consider the crystal structures of NaCl, ZnS, and CsCl (not necessarily shown in this order). a. For one of the three compounds, justify that the unit cell is consistent with stoichiometry of the compound. b. In each of the crystal structures, the cations reside in certain holes in the anions' packing structures. For each compound, what type of holes are occupied by the cations and explain why those particular types of holes are preferred.arrow_forward(2 pts) What do you expect to happen in a Na2O crystal if a Cl− ion replaces one of the O2−ions in the lattice?arrow_forward(2 pts) WSe2 is an ionic compound semiconductor that can be made to be p-type or n-type.What must happen to the chemical composition for it to be p-type? What must happen tothe chemical composition for it to be n-type?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





