
(a)
Interpretation:
The product of the reaction between actic anhydride and the given reactant is to be predicted. A complete, detailed mechanism is to be drawn if the reaction occurs.
Concept introduction:
Answer to Problem 20.36P
The product of the given reaction is
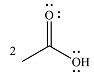
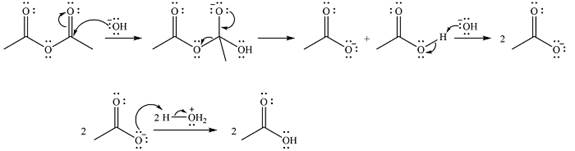
The complete mechanism of the reaction is
Explanation of Solution
The given reactant is NaOH followed by
Thus, the product of the reaction will be

The reaction will start with the nucleophilic addition of hydroxide ion from NaOH, producing a tetrahedral intermediate.
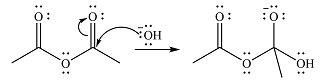
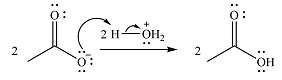
In the second step, one lone pair from negatively charged oxygen will move back to the carbon to reform the carbonyl group and eliminate an acetate ion. This step will produce one acetate ion and one acetic acid molecule, but under the strongly basic conditions, the acid will be irreversibly deprotonated to produce two carboxylate anions.
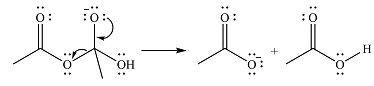
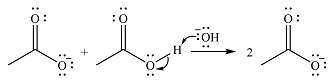
The addition of the acid (
Thus, the complete mechanism can be drawn as
The product of the reaction and its mechanism were determined based on the relative stability of the produc, and the nucleophilic addition-elimination mechanism.
(b)
Interpretation:
The product of the reaction between acetic anhydride and the given reactant is to be predicted. A complete, detailed mechanism is to be drawn if the reaction occurs.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.36P
The products of the given reaction are
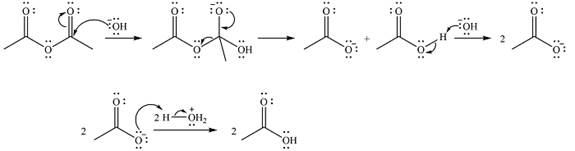
The complete mechanism of the reaction is
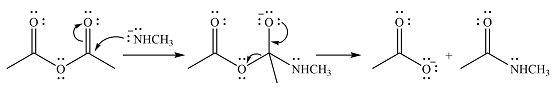
Explanation of Solution
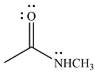
The given reactant is
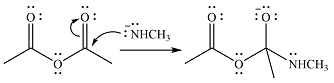
One lone pair on negatively charged oxygen will move back toward the carbon to reform the carbnyl group and eliminate an acetate ion to form the final product.
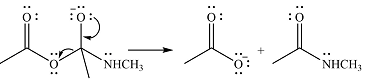
Thus, the product of the reaction will be

And the complete mechanism can be drawn as

The product of the reaction and its mechanism were dsetermined based on the relative stability of the product and the nucleophilic addition-elimination mechanism.
(c)
Interpretation:
The product of the reaction between acetic anhydride and the given reactant is to be predicted. A complete, detailed mechanism is to be drawn if the reaction occurs.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.36P
The product of the given reaction is
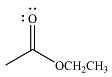
The complete mechanism of the reaction is
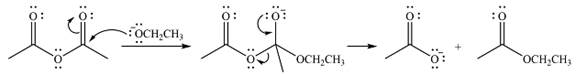
Explanation of Solution
The given reactant is
Therefore, the product of the reaction will be

In the first step, the incoming nucleophile will add to the carbonyl carbon, producing a tetrahedral intermediate.
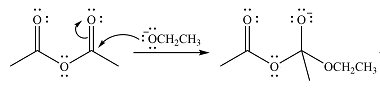
The lone pair on negativey charged ocygen will move back toward the carbon to reform the carbonyl group and eliminate acetate anion to form the final product, an ester.
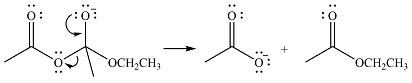
Thus, the complete mechanism can be drawn as

The product of the reaction and its mechanism were dsetermined based on the relative stability of the product and the nucleophilic addition-elimination mechanism.
(d)
Interpretation:
The product of the reaction between aceetic anhydride and the given reactant is to be predicted. A complete, detailed mechanism is to be drawn if the reaction occurs.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.36P
The product of the given reaction is
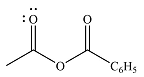
The complete mechanism of the reaction is
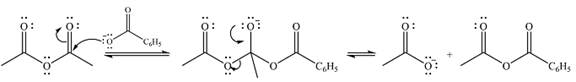
Explanation of Solution
The given reactant is
Therefore, the product of the reaction will be

In the first step, the nucleophile will add to the carbonyl carbon to produce a tetrahedral intermediate.
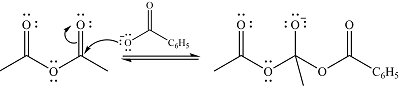
In the second step, one lone pair of negatively charged oxygen will move back to the carbon to reform the carbonyl group and eliminate acetate anion to form the final product.
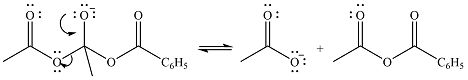
Thus, the complete mechanism can be drawn as

The product of the reaction and its mechanism were dsetermined based on the relative stability of the product and the nucleophilic addition-elimination mechanism.
(e)
Interpretation:
The product of the reaction between acetic anhydride and the given reactant is to be predicted. A complete, detailed mechanism is to be drawn if the reaction occurs.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.36P
There will be no reaction.
Explanation of Solution
The given reactant is NaBr, a source of the nucelophile
Therefore, the reaction will not occur.
Nucleophilic addition-elimination cannot occur since the possible product is of lower stability than the reactant.
(f)
Interpretation:
The product of the reaction between acetic anhydride and the given reactant is to be predicted. A complete, detailed mechanism is to be drawn if the reaction occurs.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.36P
The reaction will not occur.
Explanation of Solution
The given reactant is
Therefore, there will be no reaction.
Nucleophilic addition-elimination is not possible in this case as the nucleophile is weak and does not add to a carbonyl carbon.
(g)
Interpretation:
The product of the reaction between acetic anhydride and the given reactant is to be predicted. A complete, detailed mechanism is to be drawn if the reaction occurs.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.36P
The reaction will not occur.
Explanation of Solution
The given reactant is
Therefore, there will be no reaction.
Nucleophilic addition-elimination is not possible in this case as the given reactant is an electrophile.
(h)
Interpretation:
The product of the reaction between acetic anhydride and the given reactant is to be predicted. A complete, detailed mechanism is to be drawn if the reaction occurs.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.36P
The reaction will not occur.
Explanation of Solution
The given reactant is hexanal with an electrophilic carbon.
Therefore, there will be no reaction.
Nucleophilic addition-elimination is not possible in this case as the reactant is an electrophile.
Want to see more full solutions like this?
Chapter 20 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Germanium (Ge) is a semiconductor with a bandgap of 2.2 eV. How could you dope Ge to make it a p-type semiconductor with a larger bandgap? Group of answer choices It is impossible to dope Ge and have this result in a larger bandgap. Dope the Ge with silicon (Si) Dope the Ge with gallium (Ga) Dope the Ge with phosphorus (P)arrow_forwardWhich of the following semiconductors would you choose to have photons with the longest possible wavelengths be able to promote electrons to the semiconductor's conduction band? Group of answer choices Si Ge InSb CdSarrow_forwardWhich of the following metals is the only one with all of its bands completely full? Group of answer choices K Na Ca Alarrow_forward
- 2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); Reagents: H₂O (B); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI OH - α-α Br + enant Solvent Reagent(s) Solvent Reagent(s)arrow_forwardBased on concepts from Lecture 3-5, which of the following ionic compounds should be most soluble in water? Group of answer choices MgO BeO CaO BaOarrow_forwardFrom an energy standpoint, which two process - in the correct order - are involved in the dissolving of an ionic compound crystal? Group of answer choices Water coordination to the ions followed by sublimation into the gas phase Sublimation of the crystal into gas-phase ions followed by water coordination to the ions Ion dissociation from the crystal followed by water coordination to the ions Water coordination to the ions followed by ion dissociation from the crystalarrow_forward
- For which Group 2 metal (M), is this process the most exothermic? M2+(g) + O2−(g) + CO2(g) → MO(s) + CO2(g) Group of answer choices M = Sr M = Mg M = Ca M = Baarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); R₂BH (6); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI Solvent Reagent(s) Solvent Reagent(s) HO OHarrow_forwardFor which of the following ionic compounds would you expect the smallest difference between its theoretical and experimental lattice enthalpies? (You may assume these all have the same unit cell structure.) Electronegativities: Ca (1.0), Fe (1.8), Mg (1.2), O (3.5), S (2.5), Zn (1.6) Group of answer choices ZnO MgS CaO FeSarrow_forward
- In the Born-Haber cycle for KCl crystal formation, what enthalpy component must be divided by two? Group of answer choices KCl(s) enthalpy of formation Ionization energy for K(g) K(s) sublimation enthalpy Cl2 bond dissociation enthalpyarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O₂ / HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI хот Br Solvent Reagent(s) Solvent Reagent(s)arrow_forwardWhat is the correct chemical equation for the lattice formation reaction for CaBr2? Group of answer choices Ca2+(g) + 2 Br−(g) → CaBr2(s) ½ Ca2+(g) + Br−(g) → ½ CaBr2(s) Ca(s) + Br2(l) → CaBr2(s) Ca(s) + 2 Br−(g) → CaBr2(s)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





