
Concept explainers
(a)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a primary alcohol by reacting with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
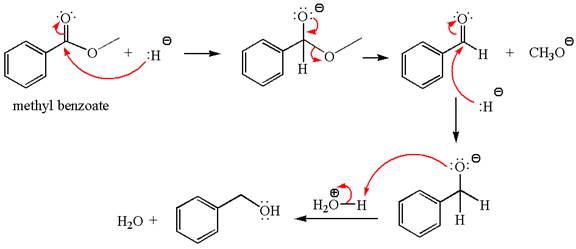
Explanation of Solution
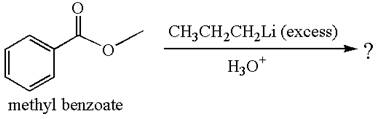
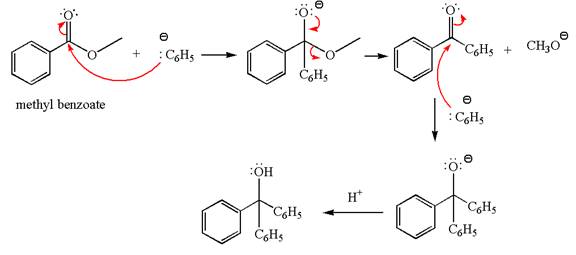
The equation for the reaction of methyl benzoate with
Methyl benzoate is an ester, which, on reaction with
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(b)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a tertiary alcohol by reacting it with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
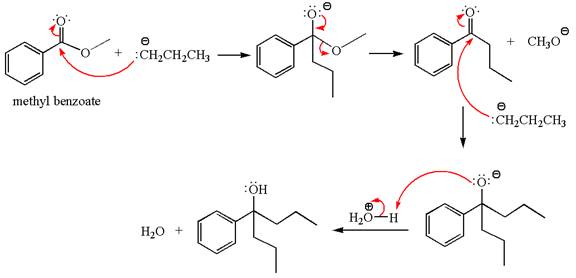
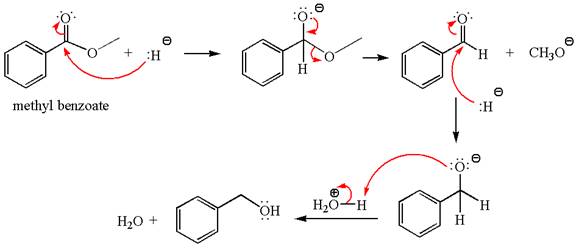
Explanation of Solution
The equation for the reaction of methyl benzoate with
Methyl benzoate is an ester, which, on reaction with
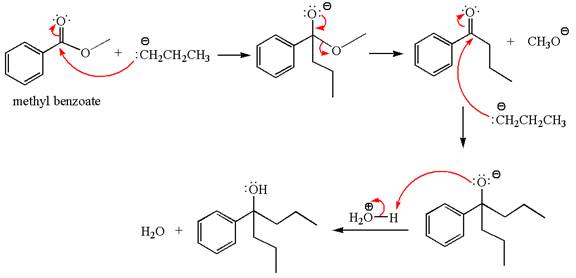
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(c)
Interpretation:
Whether methyl benzoate can react with
Concept introduction:
The reagent
Answer to Problem 20.46P
Mmethyl benzoate cannot react with
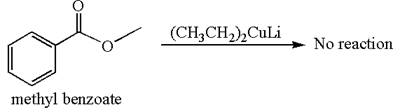
Explanation of Solution
The equation for the reaction of methyl benzoate with
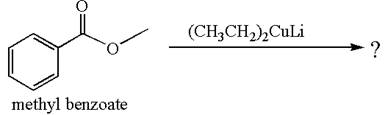
Methyl benzoate is an ester and
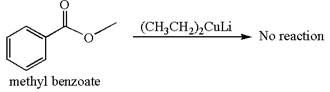
It is determined that no reaction occurs based on the reactivity of
(d)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a tertiary alcohol by reacting it with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
Explanation of Solution
The equation for the reaction of methyl benzoate with
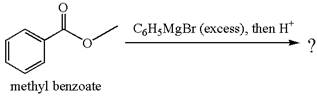
Methyl benzoate is an ester, which, on reaction with

The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(e)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be reduced to aldehyde without reducing it further to alcohol using a specific reagent such as
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
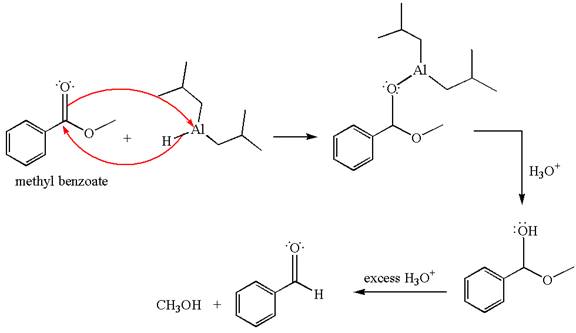
Explanation of Solution
The equation for the reaction of methyl benzoate with
Methyl benzoate is an ester, which, on reaction with
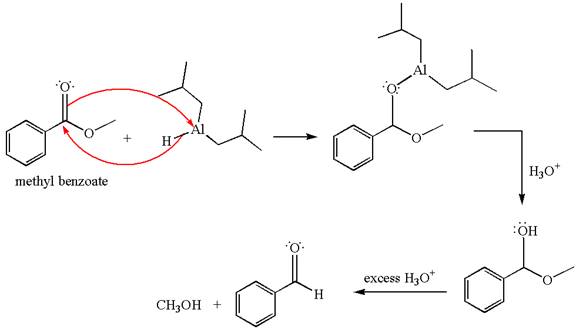
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
Want to see more full solutions like this?
Chapter 20 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Can I please get help with this question. All required information should be in data table.arrow_forwardesc For the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. Major Product: Explanation Check C ☐ + X NaOH Br F1 F2 80 F3 F4 F5 F6 1 ! @ 2 3 $ 4 % 5 Q W LU E S D A F7 * C Click and dr drawing a 2025 McGraw Hill LLC. All Rights Reserv ►II F8 4 F9 6 7 8 9 R T Y U LL F G H Jarrow_forwardCalculate equilibrium concentrations for the following reaction:N2 (g) + O2 (g) ⇋ 2 NO (g) Kc = 0.10 at 2273K initially [N2] = 0.200M; [O2] = 0.200arrow_forward
- For each scenario below, select the color of the solution using the indicator thymol blue during the titration. When you first add indicator to your Na2CO3solution, the solution is basic (pH ~10), and the color is ["", "", "", "", ""] . At the equivalence point for the titration, the moles of added HCl are equal to the moles of Na2CO3. One drop (or less!) past this is called the endpoint. The added HCl begins to titrate the thymol blue indicator itself. At the endpoint, the indicator color is ["", "", "", "", ""] . When you weren't paying attention and added too much HCl (~12 mL extra), the color is ["", "", "", "", ""] . When you really weren't paying attention and reached the second equivalence point of Na2CO3, the color isarrow_forwardThe following reaction is run in which the initial conditions include only methane (CH4) at a concentration of0.115 M. Once equilibrium was established, the concentration of acetylene (C2H2) was measured to be 0.035M. What is the value of the equilibrium constant, K?2 CH4 (g) ⇋ C2H2 (g) + 3 H2 (g)arrow_forwardCalculate the equilibrium concentration of carbon dioxide for the following reaction:2 COF2 (g) ⇋ CF4 (g) + CO2 (g) Kc = 2.00 at 10.00 °C. at equilibrium [COF2] = 0.255M; [CF4] = 0.118Marrow_forward
- In a benzene derivative that has -CH2CH3, indicate how it can be substituted by -COOH.arrow_forwardIn a sulfonated derivative of benzene, indicate how -SO3H can be eliminated.arrow_forwardWhat is the equilibrium expression (law of mass action) for the following reaction:CO2 (g) + H2O (l) ⇋ H+ (aq) + HCO3- (aq)arrow_forward
- Indicate the compound resulting from adding NaOH cyclopentane-CH2-CHO.arrow_forwardUse the provided information to calculate Kc for the following reaction at 550 °C: H2(g) + CO2(g) ⇌ CO(g) + H2O(g) Kc = ?CoO(s) + CO(g) ⇌ Co(s) + CO2(g) Kc1 = 490CoO(s) + H2(g) ⇌ Co(s) + H2O(g) Kc2 = 67arrow_forwardCalculate Kc for the reaction: I2 (g) ⇋ 2 I (g) Kp = 6.26 x 10-22 at 298Karrow_forward
