
Student's Solutions Manual for Organic Chemistry
9th Edition
ISBN: 9780134160375
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.37SP
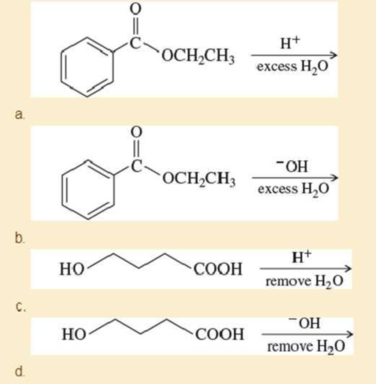
Predict the products and propose mechanisms for the following reactions.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ
Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this
system:
rise
Under these conditions, will the pressure of N2 tend to rise or fall?
☐ x10
fall
Is it possible to reverse this tendency by adding H₂?
In other words, if you said the pressure of N2 will tend to rise, can that be
changed to a tendency to fall by adding H2? Similarly, if you said the
pressure of N will tend to fall, can that be changed to a tendency to rise
by adding H₂?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of H₂ needed to reverse it.
Round your answer to 2 significant digits.
yes
no
☐
atm
Х
ด
?
olo
18
Ar
Four liters of an aqueous solution containing 6.98 mg of acetic acid were prepared. At 25°C, the measured conductivity was 5.89x10-3 mS cm-1. Calculate the degree of dissociation of the acid and its ionization constant.Molecular weights: O (15.999), C (12.011), H (1.008).Limiting molar ionic conductivities (λ+0 and λ-0) of Ac-(aq) and H+(aq): 40.9 and 349.8 S cm-2 mol-1.
Determine the change in Gibbs energy, entropy, and enthalpy at 25°C for the battery from which the data in the table were obtained.T (°C) 15 20 25 30 35Eo (mV) 227.13 224.38 221.87 219.37 216.59Data: n = 1, F = 96485 C mol–1
Chapter 20 Solutions
Student's Solutions Manual for Organic Chemistry
Ch. 20.2C - Prob. 20.1PCh. 20.2C - Name the following carboxylic acids (when...Ch. 20.4B - Rank the compounds in each set in order of...Ch. 20.5 - Prob. 20.4PCh. 20.5 - Phenols are less acidic than carboxylic acids,...Ch. 20.5 - Prob. 20.6PCh. 20.7A - Prob. 20.7PCh. 20.7B - Prob. 20.8PCh. 20.7D - Draw all four resonance forms of the fragment at...Ch. 20.7D - a. Why do most long-chain fatty acids show a large...
Ch. 20.10 - Prob. 20.13PCh. 20.10 - A carboxylic acid has two oxygen atoms, each with...Ch. 20.10 - Prob. 20.15PCh. 20.10 - The mechanism of the Fischer esterification was...Ch. 20.10 - Prob. 20.17PCh. 20.12 - Show how to synthesize the following compounds,...Ch. 20.13 - Show how you would synthesize the following...Ch. 20.14 - Prob. 20.20PCh. 20.14 - Prob. 20.21PCh. 20.15 - Propose a mechanism for the reaction of benzoic...Ch. 20.15 - Prob. 20.23PCh. 20.15 - Prob. 20.24PCh. 20 - Prob. 20.25SPCh. 20 - Give both IUPAC names and common names for the...Ch. 20 - Draw the structures of the following compounds. a....Ch. 20 - Prob. 20.28SPCh. 20 - Arrange each group of compounds in order of...Ch. 20 - Predict the products (if any) of the following...Ch. 20 - Rank the following isomers in order of increasing...Ch. 20 - Prob. 20.32SPCh. 20 - What do the following pKa values tell you about...Ch. 20 - Given the structure of ascorbic acid (vitamin C):...Ch. 20 - Prob. 20.35SPCh. 20 - Show how you would accomplish the following...Ch. 20 - Predict the products and propose mechanisms for...Ch. 20 - Prob. 20.38SPCh. 20 - Prob. 20.39SPCh. 20 - Prob. 20.40SPCh. 20 - Prob. 20.44SPCh. 20 - Prob. 20.45SPCh. 20 - Predict the major form of each compound when it is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the correct options.1. The units of the transport number are Siemens per mole.2. The Siemens and the ohm are not equivalent.3. The Van't Hoff factor is dimensionless.4. Molar conductivity does not depend on the electrolyte concentration.arrow_forwardIdeally nonpolarizable electrodes can1. participate as reducers in reactions.2. be formed only with hydrogen.3. participate as oxidizers in reactions.4. form open and closed electrochemical systems.arrow_forwardIndicate the options for an electrified interface:1. Temperature has no influence on it.2. Not all theories that describe it include a well-defined electrical double layer.3. Under favorable conditions, its differential capacitance can be determined with the help of experimental measurements.4. A component with high electronic conductivity is involved in its formation.arrow_forward
- To describe the structure of the interface, there are theories or models that can be distinguished by:1. calculation of the charge density.2. distribution of ions in the solution.3. experimentally measured potential difference.4. external Helmoltz plane.arrow_forwardIndicate the correct options when referring to Luther's equation:1. It is not always easy to compare its results with experimental results.2. It depends on the number of electrons exchanged in the species involved.3. Its foundation is thermodynamic.4. The values calculated with it do not depend on temperature.arrow_forwardIndicate which of the unit options correspond to a measurement of current density.1. A s m-22. mC s-1 m-23. Ω m-24. V J-1 m-2arrow_forward
- Indicate the options that are true when referring to electrode membranes:1. The Donnan potential, in general, does not always intervene in membranes.2. There are several ways to classify the same membrane.3. Any membrane can be used to determine the pH of a solution.4. Only one solution and one membrane are needed to determine the pH of that solution.arrow_forwardCalculate the maximum volume of carbon dioxide gasarrow_forwardIn galvanic cells, their potential1. can be measured with a potentiometer2. does not depend on the equilibrium constant of the reaction occurring within them3. is only calculated from the normal potentials of the electrodes they comprise4. can sometimes be considered a variation in a potential differencearrow_forward
- If some molecules in an excited state collide with other molecules in a ground state, this process1. can occur in solution and in the gas phase.2. can be treated as a bimolecular process.3. always results in collisional deactivation.4. does not compete with any other process.arrow_forwardRadiation of frequency v is incident on molecules in their ground state. The expected outcome is that1. the molecules do not change their state.2. the molecules transition to an excited state.3. the molecules undergo a secondary process.4. collisional deactivation occurs.arrow_forwardPredict the major product of the following reaction and then draw a curved arrow mechanism for its formation. Part: 0/2 Part 1 of 2 H₂SO heat : OH 90 Draw the structure of the major product. Click and drag to start drawing a structure. 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License