
CHEMISTRY:CENTRAL SCIENCE-W/MOD.ACCESS
14th Edition
ISBN: 9780134809694
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 6E
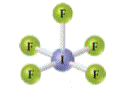
Write the chemical formula for the following compound. Is the compound ionic or molecular?
Name the compound. [Sections 2.6 and 2.8 ]
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculate the packing factor of CaTiO3.
It has a perovskite structure.
Data:
ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm;
lattice constant is a = 2(rTi4+ + ro2-).
Ca2+
02-
T14+
Consider the ions as rigid spheres.
1. 0.581 or 58.1%
2. -0.581 or -58.1 %
3. 0.254 or 25.4%
General formula ether
Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!
Please correct answer and don't used hand raiting
Chapter 2 Solutions
CHEMISTRY:CENTRAL SCIENCE-W/MOD.ACCESS
Ch. 2.3 - Which of the following factors determines the size...Ch. 2.3 - Practice Exercise 2 The diameter of a cartoon atom...Ch. 2.3 - Practice Exercise 1 Which of these atoms has the...Ch. 2.3 - Practice Exercise 2
How many protons, neutrons,...Ch. 2.3 - Prob. 2.3.1PECh. 2.3 - Practice Exercise 2
Give the complete chemical...Ch. 2.4 - Practice Exercise 1 There are two stable isotopes...Ch. 2.4 - Practice Exercise 2
Three isotopes of silicon...Ch. 2.5 - Practice Exercise 1 A biochemist who is studying...Ch. 2.5 - Practice Exercise 2 Locate Na (sodium) and Br...
Ch. 2.6 - Practice Exercise 1 Tetra carbon dioxide is an...Ch. 2.6 - Practice Exercise 2 Give the empirical formula for...Ch. 2.7 - Practice Exercise 1 In which of the following...Ch. 2.7 - Practice Exercise 2 How many protons, neutrons,...Ch. 2.7 - Practice Exercise 1
Although it is helpful to...Ch. 2.7 - Prob. 2.8.2PECh. 2.7 - Prob. 2.9.1PECh. 2.7 - Prob. 2.9.2PECh. 2.7 - Prob. 2.10.1PECh. 2.7 - Practice Exercise 2
Write the empirical formula...Ch. 2.8 - Practice Exercise 1 Which of the follow-mg ox...Ch. 2.8 - Prob. 2.11.2PECh. 2.8 - Prob. 2.12.1PECh. 2.8 - Prob. 2.12.2PECh. 2.8 - Prob. 2.13.1PECh. 2.8 - Prob. 2.13.2PECh. 2.8 - Prob. 2.14.1PECh. 2.8 - Practice Exercise 2
Give the chemical fomi uias...Ch. 2.9 - Prob. 2.15.1PECh. 2.9 - Prob. 2.15.2PECh. 2 - Prob. 1ECh. 2 - The followmg diagram is a representation of 20...Ch. 2 - 2 3 Four of the boxes in the following periodic...Ch. 2 -
24 Does the following drawing represent a neutral...Ch. 2 - 2.5 Which of the following diagrams most likely...Ch. 2 - Write the chemical formula for the following...Ch. 2 - Prob. 7ECh. 2 - Prob. 8ECh. 2 - 2.9 Are these two compounds isomers? Explain....Ch. 2 - 2.10 In the Millikan oil-drop experiment (see...Ch. 2 - A 1.0-g sample of carbon dioxide (002) is fully...Ch. 2 - Hydrogen sulfide is composed of two elements:...Ch. 2 - A chemist finds that 30.82 g of nitrogen will...Ch. 2 - 2 . 14 In a series at exper'ments. a chemist...Ch. 2 - 215 Which of the three subatomic particles was...Ch. 2 - 2.16 An unknown particle is caused to move between...Ch. 2 - 2.17 What fraction of α particle in Rutherford’s...Ch. 2 - it 18 Millikan determined the charge on the...Ch. 2 - The radius of an atom of gold (Au) is about 1.35 Å...Ch. 2 - 220 An atom of rhodium (Rh) has a diameter of...Ch. 2 - 2.21 Answer the following questions without...Ch. 2 - Determine whether each of the following statements...Ch. 2 - Consider an atom of "B. a. How many protons,...Ch. 2 - Consider an atom of 63Cu. a. How many protons,...Ch. 2 - 2.25
3. Define atomic number and mass number
b....Ch. 2 -
2 26
Which two of the following are isotopes of...Ch. 2 - How many ptotons, neutrons, and electrons are in...Ch. 2 - 2-28 Each of the following isotopes is used in...Ch. 2 - Prob. 29ECh. 2 - Fill in the gaps in the following table, assuming...Ch. 2 - Write the correct symbol, with both superscript...Ch. 2 - One way in which Earth's evolution as a planet can...Ch. 2 - 2.33
a. What isotope is used as the standard in...Ch. 2 - 2.34
a. What is the mass in amu of a carbon-12...Ch. 2 - Only two isotopes of copper occur naturally:63Cu...Ch. 2 - 2.36 Rubidium has two naturally occurring...Ch. 2 - a. Thomson’s cathode-ray tube (Figure 2.49) and...Ch. 2 -
2.38 Consider the mass spectrometer shown in...Ch. 2 - Naturally occurring magnesium has the following...Ch. 2 - Mass spectrometry is more often applied to...Ch. 2 - 2-41 For each of the following elements, write its...Ch. 2 - Locate each of the following elements in the...Ch. 2 - 2-43 For each of the following elements, write its...Ch. 2 - 2.44 The elements of group 4A show an interesting...Ch. 2 - 2.45 The structural formulas of the compounds...Ch. 2 - 2.46 Ball-and-stick representations of benzene, a...Ch. 2 - 2447 What are the molecular and empirical formulas...Ch. 2 -
2.48 Two substances have the same molecular and...Ch. 2 - 2.49 Write the empirical formula corresponding to...Ch. 2 - Determine the molecular and empirical formulas of...Ch. 2 - 251 How many hydrogen atoms are un each of the...Ch. 2 - Prob. 52ECh. 2 - 253 Write the molecular and structural formulas...Ch. 2 - 2-54 Write the molecular and structural formulas...Ch. 2 - Fill in the gaps in the following table’Ch. 2 - 2.56 Fill in the gaps in the following...Ch. 2 - Each of the following elements is capable of...Ch. 2 - Using the periodic table, predict the charges of...Ch. 2 - 2.59 Using the periodic table to guide you,...Ch. 2 - 2-60 The most common charge associated with...Ch. 2 - 2.61 Predict the chemical formula for the ionic...Ch. 2 - Predict the chemical formulas of the compounds...Ch. 2 - Prob. 63ECh. 2 - Prob. 64ECh. 2 - Predict whether each of the following compounds is...Ch. 2 - 2.66 Which of the following are ionic, and which...Ch. 2 - Prob. 67ECh. 2 - Prob. 68ECh. 2 -
2.69 Give the names and charges of the cation and...Ch. 2 - Give the names and charges of the cation and anion...Ch. 2 -
2.71 Name the following ionic compounds:
a....Ch. 2 - Prob. 72ECh. 2 -
2.73 Write the chemical formulas for the...Ch. 2 -
Give the chemical formula for each of the...Ch. 2 -
2.75 Give the name or chemical formula, as...Ch. 2 - Prob. 76ECh. 2 -
2.T Give the name or Chemical formula, as...Ch. 2 - The oxides of nitrogen are very important...Ch. 2 - Prob. 79ECh. 2 - Assume that you encounter the following sentences...Ch. 2 - a. What is a hydrocarbon? b. Pentane is the alkane...Ch. 2 - 2.82
a. What is meant by the term isomer?
b. Among...Ch. 2 -
2.83
What is a functional group?
What functional...Ch. 2 -
2.84 Consider the following organic substances:...Ch. 2 -
2.85 Chloropropane is derived from propane by...Ch. 2 -
2.86 Draw the structural formulas for three...Ch. 2 - Suppose a scientist repeats the Millikan oil-drop...Ch. 2 -
2.88 The natural abundance of 3He is...Ch. 2 - A cube of gold that is 1.00 cm on a side has a...Ch. 2 -
2.90 The diameter of a rubidium atom is 4.95 A....Ch. 2 -
2.91
Assuming the dimensions of the nucleus and...Ch. 2 -
2.92 Identify the element reoresented by the each...Ch. 2 -
2.93 The nucleus of 6Li is a powerful absorber of...Ch. 2 - The element oxygen has three naturally occurring...Ch. 2 - The element lead (Pb) consists of four naturally...Ch. 2 -
2.96 Gallium (Ga) consists of two naturally...Ch. 2 - Using a suitable reference such as the CRC...Ch. 2 - There are two different isotopes of bromine atoms....Ch. 2 -
2.99 It is common in mass spectrometry to assume...Ch. 2 - From the following list of elements—Ar, H, Ga, Al,...Ch. 2 -
2.101 The first atoms of seaborgium (Sg) were...Ch. 2 -
2.102 The explosion of an atomic bomb releases...Ch. 2 -
2.103. A U.S. 1-cent coin (a penny) has a...Ch. 2 -
2.104 The U.S. Mint produces a dollar coin called...Ch. 2 -
2.105 From the molecular structures shown here,...Ch. 2 -
2.106 Name each of the following oxides. Assuming...Ch. 2 - Prob. 107AECh. 2 -
2.108 Cyclopropane is an interesting hydrocarbon....Ch. 2 - Prob. 109AECh. 2 - Prob. 110AECh. 2 - Give the chemical names of each of the following...Ch. 2 -
2.112 Many familiar substances have common,...Ch. 2 -
2.113 Because many ions and compounds have very...Ch. 2 -
2.114 In what part of the atom does the strong...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forward
- In the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forwardThe degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forwardIn natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forward
- Most ceramic materials have low thermal conductivities because:(a) Electron mobility is strongly restricted due to their strong ionic-covalent bonding.(b) False, in general they are excellent thermal conductors (they are used in ovens).(c) Electron mobility is dependent on T and therefore they are poor conductors at high temperatures.(d) Electron mobility is very restricted by secondary bonds.arrow_forwardResistivity and electrical conductivity.(a) In metals, resistivity decreases.(b) In metals, resistivity decreases and conductivity in semiconductors also decreases with increasing temperature.(c) With increasing temperature, resistivity in metals and conductivity in semiconductors also increases.(d) None of the above.arrow_forwardState the difference between concrete and Portland cement.(a) There are no differences, in concrete the chemical composition is silicates and in cement aluminates.(b) The chemical composition of concrete is based on silicates and in cement aluminates.(c) Concrete is composed of aggregates bound by cement and cement "only" contains different minerals.(d) Cement is aggregates bound by concrete.arrow_forward
- Amorphous polymers are usually transparent and semi-crystalline polymers are usually opaque. Correct?(a) No. They are all made up of polymer chains. True if they were monomers.(b) Yes. The arrangement of the chains determines the passage of light.(c) No. It is the other way around.(d) Crystallinity or amorphousness does not affect the transparency or opacity of the material.arrow_forwardThe name ferrites refers to a family of(a) ceramic materials that exhibit ferrimagnetic behavior due to their ionic composition.(b) polymeric materials that exhibit ferrimagnetic behavior due to their ionic composition.(c) concrete-based materials that exhibit ferrimagnetic behavior due to their ionic composition.(d) superconducting materials that exhibit ferrimagnetic behavior due to their ionic composition.arrow_forwardState the two main factors affecting ion packing in the solid state.(a) Number of covalent bonds and their unsaturation.(b) Mechanical properties and degradation temperature.(c) Number of crystalline phases present and grain size.(d) Electroneutrality and ion size.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
NEET Chemistry | Group 14 Carbon Family | Theory & Problem Solving | In English | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=enOGIrcHh54;License: Standard YouTube License, CC-BY