
Connect Online Access 1-Semester for Organic Chemistry
6th Edition
ISBN: 9781260475609
Author: SMITH, Janice
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 64P
Use the principles in Section 2.5 to label the most acidic hydrogen in each drug.
Explain your choice.
a. 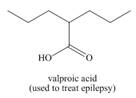 b.
b. 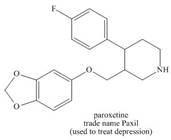 c.
c. 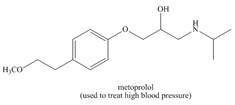
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q3: Describes the relationship (identical, constitutional isomers, enantiomers or diastereomers)
of each pair of compounds below.
ག
H
CH3
OH
OH
CH3
H3C
OH
OH
OH
//////////
C
CH3
CH3
CH3
CH3
H3C
CH 3
C/III.....
Physics & Astronomy
www.physics.northweste
COOH
H
нош.....
H
2
OH
HO
CH3
HOOC
H
CH3
CH3
CH3
Br.
H
H
Br
and
H
H
H
H
Q1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label
each compound as chiral or achiral.
OH
HO
CI
Br
H
CI
CI
Br
CI
CI
Xf x f g
Br
D
OH
Br
Br
H₂N
R.
IN
Ill
I
-N
S
OMe
D
II
H
CO₂H
1/111
DuckDuckG
These are synthesis questions. You need to show how the starting material can be converted into
the product(s) shown. You may use any reactions we have learned. Show all the reagents you
need. Show each molecule synthesized along the way and be sure to pay attention to the
regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made
along the way, you need to draw both enantiomers and label the mixture as "racemic".
All of the carbon atoms of the products must come from the starting material!
?
H
H
Chapter 2 Solutions
Connect Online Access 1-Semester for Organic Chemistry
Ch. 2.1 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2.2 - a. Draw the conjugate acid of each base:...Ch. 2.2 - Label each statement as True or False.
a. is the...Ch. 2.2 - Decide which compound is the acid and which is the...Ch. 2.2 - Draw the products formed from the acid-base...Ch. 2.3 - Which compound in each pair is the stronger acid?...Ch. 2.3 - Use a calculator when necessary to answer the...Ch. 2.3 - Rank the conjugate bases of each of group of acids...Ch. 2.3 - Problem-2.10 Considers two acids: (formic acid,)...Ch. 2.3 - Prob. 11P
Ch. 2.4 - Draw the products of each reaction and determine...Ch. 2.4 - Prob. 13PCh. 2.5 - Without reference to a pKa table, decide which...Ch. 2.5 - Rank the labeled H atoms in the following compound...Ch. 2.5 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2.5 - Which compound in each pair is the stronger acid?...Ch. 2.5 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2.5 - Explain the apparent paradox. HBr is a stronger...Ch. 2.5 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2.5 - Prob. 23PCh. 2.5 - For each pair of compounds: [1] Which indicated H...Ch. 2.5 - Rank the compounds in each group in order of...Ch. 2.5 - Prob. 26PCh. 2.5 - Prob. 27PCh. 2.6 - Prob. 28PCh. 2.7 - Problem 2.29
Compounds like amphetamine that...Ch. 2.8 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2.8 - Which species are Lewis acids?
a. b. c. d.
Ch. 2.8 - For each reaction, label the Lewis acid and base....Ch. 2.8 - Prob. 33PCh. 2.8 - Prob. 34PCh. 2.8 - Label the Lewis acid and base. Use curved arrow...Ch. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - 2.38 What is the conjugate acid of each base?
a....Ch. 2 - 2.39 What is the conjugate base of each acid?
a....Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Prob. 43PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Draw the products of each reaction. Use the pKa...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - 2.59 Atenolol is a (beta) blocker, a drug used to...Ch. 2 - 2.60 Use the principles in Section 2.5 to label...Ch. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 66PCh. 2 - 2.63 Classify each compound as a Lewis base, a...Ch. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Label the Lewis acid and Lewis base in each...Ch. 2 - 2.66 Draw the products of each Lewis acid-base...Ch. 2 - Prob. 71PCh. 2 - 2.68 Answer the following questions about the four...Ch. 2 - Prob. 73PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - Prob. 76PCh. 2 - Prob. 77PCh. 2 - Prob. 82P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q5: Draw every stereoisomer for 1-bromo-2-chloro-1,2-difluorocyclopentane. Clearly show stereochemistry by drawing the wedge-and-dashed bonds. Describe the relationship between each pair of the stereoisomers you have drawn.arrow_forwardClassify each pair of molecules according to whether or not they can participate in hydrogen bonding with one another. Participate in hydrogen bonding CH3COCH3 and CH3COCH2CH3 H2O and (CH3CH2)2CO CH3COCH3 and CH₂ CHO Answer Bank Do not participate in hydrogen bonding CH3CH2OH and HCHO CH3COCH2CH3 and CH3OHarrow_forwardNonearrow_forward
- Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 4A (g) + 2B (g) → 2C (g) + 7D (g) AHrxn =?kJ Substance AH in kJ/mol A (g) - 20.42 B (g) + 32.18 C (g) - 72.51 D (g) - 17.87arrow_forwardDetermine ASran for Zn(s) + 2HCl(aq) = ZnCl2(aq) + H2(aq) given the following information: Standard Entropy Values of Various Substance Substance So (J/mol • K) 60.9 Zn(s) HCl(aq) 56.5 130.58 H2(g) Zn2+(aq) -106.5 55.10 CI (aq)arrow_forward3) Catalytic hydrogenation of the compound below produced the expected product. However, a byproduct with molecular formula C10H12O is also formed in small quantities. What is the by product?arrow_forward
- What is the ΔHorxn of the reaction? NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq) ΔHorxn 1= ________ kJ/molarrow_forward= +92kJ ΔΗ = +170kJ Use the following reactions: 2NH3(9) N2(g) + 3H2(g) → 11/N2(g) + 2H2O (1) → NO2(g) + 2H2(g) Determine the DH° of this reaction: NO2(g) + H2(g) → 2(g) → 2H2O(l) + NH3(9) ΔΗarrow_forwardDetermine the entropy change for the reaction SO2(g) + O2(g) following information: Standard Entropy Values of Various Substance Substance SO2(g) 02(g) SO3(g) So (J/mol K) 248.2 205.0 256.8 → SO3(g) given thearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY