
BELK SCIENCE F/LIFE-MASTRG. BIOL.AC+EBK
6th Edition
ISBN: 9781323907634
Author: BELK
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 3LTB
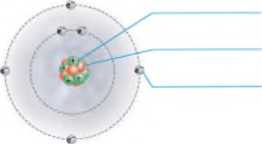
Add labels to the figure that follows, which illustrates the subatomic particles associated with a carbon atom.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The configuration of the given structure below is: *
но-сн, о он
но
CH,-OH
OH
alpha-L
alpha-D
beta-L
beta-D
Draw a “dot & cross” diagram to show the shape of a water molecule (H2O). Bond angles need to be shown. State the shape of a water molecule.
The configuration of the given structure below is:"
CH2OH
он
ÓH
O alpha-L
alpha-D
O beta -L
O beta-D
Chapter 2 Solutions
BELK SCIENCE F/LIFE-MASTRG. BIOL.AC+EBK
Ch. 2 - Prob. 1LTBCh. 2 - Prob. 2LTBCh. 2 - Add labels to the figure that follows, which...Ch. 2 - Prob. 4LTBCh. 2 - Prob. 5LTBCh. 2 - Which of the following terms is least like the...Ch. 2 - Different proteins are composed of different...Ch. 2 - Proteins may function as ___________ genetic...Ch. 2 - Prob. 9LTBCh. 2 - Eukaryotic cells differ from prokaryotic cells in...
Ch. 2 - Which of the following lists the chemical bonds...Ch. 2 - Which of the following is not consistent with...Ch. 2 - Consider a virus composed of a protein coat...Ch. 2 - Prob. 2AAATBCh. 2 - Carbon, oxygen, hydrogen, and nitrogen are common...Ch. 2 - List some alternate explanations that should be...Ch. 2 - Do some web-based research using scientifically...Ch. 2 - Prob. 1MTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Suggest to biochemistryarrow_forwardA protein is composed of amino acids, which are held together by the covalent bonds known as: phosphodiester bonds ester bonds glycosidic bonds ether bonds peptide bondsarrow_forwardIdentify the molecule shown below. You may also see the molecule by clicking on this link. H H HO O phospholipid O DNA ORNA O cholesterol O peptide (protein)arrow_forward
- fill the following table polymer or large biological molecule monomer or smaller subunit one funtion name od covalent bond nulceic acids three fatty acids easter bond acts as an enzyme immediate or long-term energy source glycosidic linkagearrow_forwardIdentify the elements that make up the following biomolecules. Write a yes or no in the box to indicate if the element is present or not. BIOMOLECULE carbohydrates proteins lipids carbon hydrogen nitrogen oxygen phosphorus nucleic acidsarrow_forwardThe “octet rule” in chemistry helps predict the types of bonds thatatoms will form. In general, an atom will be most stable if it fills itsouter shell of 8 electrons. Atoms with fewer than 4 valence electronstend to donate electrons and those with more than 4 valence electronstend to accept additional electrons; those with exactly 4 can do both.Using this rule, determine what category each of the followingelements falls into: N, S, C, P, O, H, Ca, Fe, and Mg. (You will needto work out the valence of the atoms.)arrow_forward
- Which of the following combinations describes the electron in the modern model of an atom? O negative charge and located in orbitals surrounding the nucleus Opositive charge and located in orbitals surrounding the nucleus negative charge and located in the nucleus Ono charge and located in the nucleusarrow_forwardIndicate whether the statement below is true or false.Any covalently bonded H atom can participate in a hydrogen bond if it comes in close proximity with an oxygen atom that forms part of a water molecule.arrow_forwardIdentify if the molecule below is a protein, lipid, nucleic acid or carbohydrate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning



Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license