
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 39P
What is the conjugate base of each acid?
a.
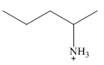 c.
c. 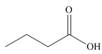 d.
d. 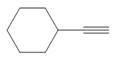
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
write IUPAC names for these alcohols
Please list the String of Letters in the correct order.
2. Propose an efficient synthesis for each of the following transformations. Pay
careful attention to both the regio and stereochemical outcomes. ¡
H H
racemic
Chapter 2 Solutions
ORGANIC CHEMISTRY
Ch. 2.1 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2.2 - a. Draw the conjugate acid of each base:...Ch. 2.2 - Label each statement as True or False.
a. is the...Ch. 2.2 - Decide which compound is the acid and which is the...Ch. 2.2 - Draw the products formed from the acid-base...Ch. 2.3 - Which compound in each pair is the stronger acid?...Ch. 2.3 - Use a calculator when necessary to answer the...Ch. 2.3 - Rank the conjugate bases of each of group of acids...Ch. 2.3 - Problem-2.10 Considers two acids: (formic acid,)...Ch. 2.3 - Prob. 11P
Ch. 2.4 - Draw the products of each reaction and determine...Ch. 2.4 - Prob. 13PCh. 2.5 - Without reference to a pKa table, decide which...Ch. 2.5 - Rank the labeled H atoms in the following compound...Ch. 2.5 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2.5 - Which compound in each pair is the stronger acid?...Ch. 2.5 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2.5 - Explain the apparent paradox. HBr is a stronger...Ch. 2.5 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2.5 - Prob. 23PCh. 2.5 - For each pair of compounds: [1] Which indicated H...Ch. 2.5 - Rank the compounds in each group in order of...Ch. 2.5 - Prob. 26PCh. 2.5 - Prob. 27PCh. 2.6 - Prob. 28PCh. 2.7 - Problem 2.29
Compounds like amphetamine that...Ch. 2.8 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2.8 - Which species are Lewis acids?
a. b. c. d.
Ch. 2.8 - For each reaction, label the Lewis acid and base....Ch. 2.8 - Prob. 33PCh. 2.8 - Prob. 34PCh. 2.8 - Label the Lewis acid and base. Use curved arrow...Ch. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - 2.38 What is the conjugate acid of each base?
a....Ch. 2 - 2.39 What is the conjugate base of each acid?
a....Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Prob. 43PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Draw the products of each reaction. Use the pKa...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - 2.59 Atenolol is a (beta) blocker, a drug used to...Ch. 2 - 2.60 Use the principles in Section 2.5 to label...Ch. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 66PCh. 2 - 2.63 Classify each compound as a Lewis base, a...Ch. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Label the Lewis acid and Lewis base in each...Ch. 2 - 2.66 Draw the products of each Lewis acid-base...Ch. 2 - Prob. 71PCh. 2 - 2.68 Answer the following questions about the four...Ch. 2 - Prob. 73PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - Prob. 76PCh. 2 - Prob. 77PCh. 2 - Prob. 82P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Zeroth Order Reaction In a certain experiment the decomposition of hydrogen iodide on finely divided gold is zeroth order with respect to HI. 2HI(g) Au H2(g) + 12(9) Rate = -d[HI]/dt k = 2.00x104 mol L-1 s-1 If the experiment has an initial HI concentration of 0.460 mol/L, what is the concentration of HI after 28.0 minutes? 1 pts Submit Answer Tries 0/5 How long will it take for all of the HI to decompose? 1 pts Submit Answer Tries 0/5 What is the rate of formation of H2 16.0 minutes after the reaction is initiated? 1 pts Submit Answer Tries 0/5arrow_forwardangelarodriguezmunoz149@gmail.com Hi i need help with this question i am not sure what the right answers are.arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Don't used hand raitingarrow_forwardDon't used Ai solutionarrow_forwardSaved v Question: I've done both of the graphs and generated an equation from excel, I just need help explaining A-B. Below is just the information I used to get the graphs obtain the graph please help. Prepare two graphs, the first with the percent transmission on the vertical axis and concentration on the horizontal axis and the second with absorption on the vertical axis and concentration on the horizontal axis. Solution # Unknown Concentration (mol/L) Transmittance Absorption 9.88x101 635 0.17 1.98x101 47% 0.33 2.95x101 31% 0.51 3.95x10 21% 0.68 4.94x10 14% 24% 0.85 0.62 A.) Give an equation that relates either the % transmission or the absorption to the concentration. Explain how you arrived at your equation. B.) What is the relationship between the percent transmission and the absorption? C.) Determine the concentration of the ironlll) salicylate in the unknown directly from the graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight…arrow_forward
- Don't used Ai solutionarrow_forwardCalculate the differences between energy levels in J, Einstein's coefficients of estimated absorption and spontaneous emission and life time media for typical electronic transmissions (vnm = 1015 s-1) and vibrations (vnm = 1013 s-1) . Assume that the dipolar transition moments for these transactions are in the order of 1 D.Data: 1D = 3.33564x10-30 C m; epsilon0 = 8.85419x10-12 C2m-1J-1arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY