
Concept explainers
Interpretation: All the lone pairs for each of the following compounds should be drawn.
Concept Introduction The unshared pair of electrons are said to be lone pairs of electrons that are present in an atom of a compound.
Answer to Problem 34PP
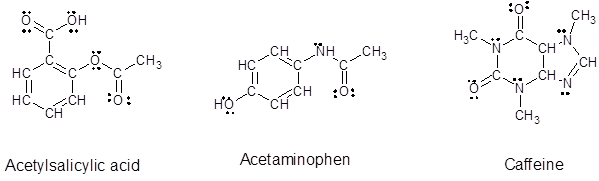
Explanation of Solution
The number of valence electrons in each isolated atom present in given compound are:
For Carbon = 4
For Hydrogen = 1
For Nitrogen = 5
For Oxygen = 6
Identify the number of bonds in the given compound:
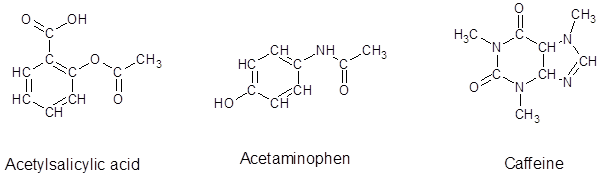
From the given structure of acetylsalicylic acid, each carbon atoms forms four bonds, hydrogen atoms forms one bond and oxygen atoms forms two bonds.
From the given structure of acetaminophen, each carbon atoms forms four bonds, hydrogen atoms forms one bond, oxygen atoms forms two bonds and nitrogen atom forms three bonds.
From the given structure of caffeine, each carbon atoms forms four bonds, hydrogen atoms forms one bond, oxygen atoms forms two bonds and nitrogen atom forms three bonds.
Since, all the valence electrons are involved in bond formation for carbon and hydrogen so there are no lone pair electrons on them. For all the oxygen atoms that forms two bonds uses two valence electrons out of six to form bond and rest four electrons are present as two lone-pair on each oxygen atom. For all the nitrogen atoms that forms three bonds uses three valence electrons out of five to form bond and rest two electrons are present as a lone-pair on each nitrogen atom. So, all the lone pairs for each compound are:
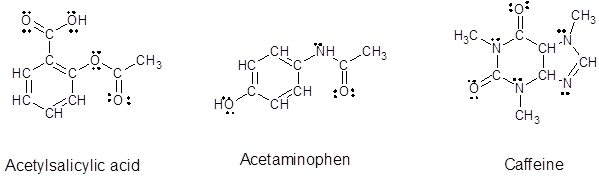
Want to see more full solutions like this?
Chapter 2 Solutions
ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG(LL)
- Assign all the protonsarrow_forwardAssign all the carbonsarrow_forward9 7 8 C 9 8 200 190 B 5 A -197.72 9 8 7 15 4 3 0: ང་ 200 190 180 147.52 134.98 170 160 150 140 130 120 110 100 90 90 OH 10 4 3 1 2 -143.04 140. 180 170 160 150 140 130 120 110 100 90 CI 3 5 1 2 141.89 140.07 200 190 180 170 160 150 140 130 120 110 100 ៖- 90 129. 126.25 80 70 60 -60 50 40 10 125.19 -129.21 80 70 3.0 20 20 -8 60 50 10 ppm -20 40 128.31 80 80 70 60 50 40 40 -70.27 3.0 20 10 ppm 00˚0-- 77.17 30 20 20 -45.36 10 ppm -0.00 26.48 22.32 ―30.10 ―-0.00arrow_forward
- Assign all the carbonsarrow_forwardC 5 4 3 CI 2 the Righ B A 5 4 3 The Lich. OH 10 4 5 3 1 LOOP- -147.52 T 77.17 -45.36 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 ppm B -126.25 77.03 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 ppm 200 190 180 170 160 150 140 130 120 110 100 90 80 TO LL <-50.00 70 60 50 40 30 20 10 ppm 45.06 30.18 -26.45 22.36 --0.00 45.07 7.5 1.93 2.05 -30.24 -22.36 C A 7 8 5 ° 4 3 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 8 5 4 3 ཡི་ OH 10 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 5 4 3 2 that th 7 I 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 115 2.21 4.00 1.0 ppm 6.96 2.76 5.01 1.0 ppm 6.30 1.00arrow_forwardCurved arrows were used to generate the significant resonance structure and labeled the most significant contribute. What are the errors in these resonance mechanisms. Draw out the correct resonance mechanisms with an brief explanation.arrow_forward
- What are the: нсе * Moles of Hice while given: a) 10.0 ml 2.7M ? 6) 10.ome 12M ?arrow_forwardYou are asked to use curved arrows to generate the significant resonance structures for the following series of compounds and to label the most significant contributor. Identify the errors that would occur if you do not expand the Lewis structures or double-check the mechanisms. Also provide the correct answers.arrow_forwardhow to get limiting reactant and % yield based off this data Compound Mass 6) Volume(mL Ben zaphone-5008 ne Acetic Acid 1. Sam L 2-propanot 8.00 Benzopin- a col 030445 Benzopin a Colone 0.06743 Results Compound Melting Point (°c) Benzopin acol 172°c - 175.8 °c Benzoping to lone 1797-180.9arrow_forward
- Assign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forwardAssign the functional group bands on the IR spectra.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





