
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.77P
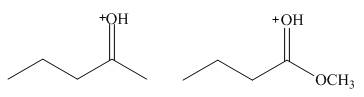
Which compound, M or N, is the stronger acid? Explain your choice.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the major enolate formed when treated with LDA? And why that one?
4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following
compounds.
a.
HH :D:
+1
I
H-N-C-C-O-H
I
H
b.
HH H
Н
:N=C-C-C=C-CEC-H
:0:
total o
H-C-H
H-C = `C-H
I
H.
11
H-C = C=
CH
H
total o
total π
total π
1
H
In the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJ
Chapter 2 Solutions
Organic Chemistry-Package(Custom)
Ch. 2 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2 - a. Draw the conjugate acid of each base:...Ch. 2 - Label the acid and base, and the conjugate acid...Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Which compound in each pair is the stronger acid?...Ch. 2 - Use a calculator when necessary to answer the...Ch. 2 - Rank the conjugate bases of each of group of acids...Ch. 2 - Problem-2.10 Considers two acids: (formic acid,)...Ch. 2 - Estimate the pKa of each of the indicated bonds.
Ch. 2 - Draw the products of each reaction and determine...Ch. 2 - Prob. 2.12PCh. 2 - Without reference to a pKa table, decide which...Ch. 2 - which compound in each pair of isomers is the...Ch. 2 - Prob. 2.15PCh. 2 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2 - whichcompound in each pair is the stronger acid? a...Ch. 2 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2 - Explain the apparent paradox. HBr is a stronger...Ch. 2 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2 - Acetonitrile (CH3CN) has a pKa of 25, making it...Ch. 2 - For each pair of compounds: [1] Which indicated H...Ch. 2 - Rank the compounds in each group in order of...Ch. 2 - Which proton in each of the following drugs is...Ch. 2 - Prob. 2.25PCh. 2 - Problem 2.29
Compounds like amphetamine that...Ch. 2 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2 - Which species are Lewis acids?
a. b. c. d.
Ch. 2 - For each reaction, label the Lewis acid and base....Ch. 2 - Prob. 2.30PCh. 2 - Prob. 2.31PCh. 2 - Prob. 2.32PCh. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - Prob. 2.35PCh. 2 - Prob. 2.36PCh. 2 - a Draw the conjugate acid of ethylene, CH2 = CH2....Ch. 2 - 2.40 Draw the products formed from the acid-base...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Prob. 2.40PCh. 2 - Draw the product of acid-base reaction. a. c. b....Ch. 2 - Prob. 2.42PCh. 2 - Prob. 2.43PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Which compounds can be deprotonated by OH, so that...Ch. 2 - Draw the product of each reaction. Use the pKa...Ch. 2 - Rank the following compounds in order of...Ch. 2 - Rank the following ions in order of increasing...Ch. 2 - Prob. 2.51PCh. 2 - Prob. 2.52PCh. 2 - The pKa of three CH bonds is given below. a. For...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - 2.56 Draw the structure of a constitutional isomer...Ch. 2 - 2.57 Many drugs are Bronsted-Lowry acids or...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - Prob. 2.58PCh. 2 - Ethyl butanoate, CH3CH2CH2CO2CH2CH3, is one of the...Ch. 2 - Prob. 2.60PCh. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 2.62PCh. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Prob. 2.64PCh. 2 - Draw the products of each Lewis acid-base...Ch. 2 - Prob. 2.66PCh. 2 - Prob. 2.67PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - Prob. 2.70PCh. 2 - 2.72 DBU, is a base we will encounter in...Ch. 2 - 2.73 Molecules like acetamide can be protonated...Ch. 2 - Two pKa values are reported for malonic acid, a...Ch. 2 - Prob. 2.74PCh. 2 - 2.76 Write a stepwise reaction sequence using...Ch. 2 - Prob. 2.76PCh. 2 - 2.78 Which compound, M or N, is the stronger acid?...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the processes in the dismutation of Cu2O.arrow_forward1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forwarddraw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forward
- Draw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forwardPost Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forward
- Indicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forwardHow can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?arrow_forwardHow many mL of H2O2 from the 30% bottle must be collected to prepare 6 mL of 6% H2O2.arrow_forward
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY