
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.38P
Draw the products formed from the acid-base reaction of H2SO4with each
compound.
a.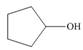 b.
b.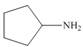 c.
c.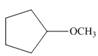 d.
d.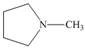
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify the glycosidic linkage.
Draw the mechanism for the substitution reaction converting an alcohol into an alkyl halide. If chirality is important to the reaction include it.
Write, in words three different reactions we can use to make an alcohol.
Chapter 2 Solutions
Organic Chemistry-Package(Custom)
Ch. 2 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2 - a. Draw the conjugate acid of each base:...Ch. 2 - Label the acid and base, and the conjugate acid...Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Which compound in each pair is the stronger acid?...Ch. 2 - Use a calculator when necessary to answer the...Ch. 2 - Rank the conjugate bases of each of group of acids...Ch. 2 - Problem-2.10 Considers two acids: (formic acid,)...Ch. 2 - Estimate the pKa of each of the indicated bonds.
Ch. 2 - Draw the products of each reaction and determine...Ch. 2 - Prob. 2.12PCh. 2 - Without reference to a pKa table, decide which...Ch. 2 - which compound in each pair of isomers is the...Ch. 2 - Prob. 2.15PCh. 2 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2 - whichcompound in each pair is the stronger acid? a...Ch. 2 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2 - Explain the apparent paradox. HBr is a stronger...Ch. 2 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2 - Acetonitrile (CH3CN) has a pKa of 25, making it...Ch. 2 - For each pair of compounds: [1] Which indicated H...Ch. 2 - Rank the compounds in each group in order of...Ch. 2 - Which proton in each of the following drugs is...Ch. 2 - Prob. 2.25PCh. 2 - Problem 2.29
Compounds like amphetamine that...Ch. 2 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2 - Which species are Lewis acids?
a. b. c. d.
Ch. 2 - For each reaction, label the Lewis acid and base....Ch. 2 - Prob. 2.30PCh. 2 - Prob. 2.31PCh. 2 - Prob. 2.32PCh. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - Prob. 2.35PCh. 2 - Prob. 2.36PCh. 2 - a Draw the conjugate acid of ethylene, CH2 = CH2....Ch. 2 - 2.40 Draw the products formed from the acid-base...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Prob. 2.40PCh. 2 - Draw the product of acid-base reaction. a. c. b....Ch. 2 - Prob. 2.42PCh. 2 - Prob. 2.43PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Which compounds can be deprotonated by OH, so that...Ch. 2 - Draw the product of each reaction. Use the pKa...Ch. 2 - Rank the following compounds in order of...Ch. 2 - Rank the following ions in order of increasing...Ch. 2 - Prob. 2.51PCh. 2 - Prob. 2.52PCh. 2 - The pKa of three CH bonds is given below. a. For...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - 2.56 Draw the structure of a constitutional isomer...Ch. 2 - 2.57 Many drugs are Bronsted-Lowry acids or...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - Prob. 2.58PCh. 2 - Ethyl butanoate, CH3CH2CH2CO2CH2CH3, is one of the...Ch. 2 - Prob. 2.60PCh. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 2.62PCh. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Prob. 2.64PCh. 2 - Draw the products of each Lewis acid-base...Ch. 2 - Prob. 2.66PCh. 2 - Prob. 2.67PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - Prob. 2.70PCh. 2 - 2.72 DBU, is a base we will encounter in...Ch. 2 - 2.73 Molecules like acetamide can be protonated...Ch. 2 - Two pKa values are reported for malonic acid, a...Ch. 2 - Prob. 2.74PCh. 2 - 2.76 Write a stepwise reaction sequence using...Ch. 2 - Prob. 2.76PCh. 2 - 2.78 Which compound, M or N, is the stronger acid?...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forward
- Which of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forwardWhat the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forward
- Of the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forwardplease answer in the scope of the SCH4U course, I am having a hard time understanding, may you show all steps please and thank you! can you also put the final answers in the table so its understandablearrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swem oxidation or heat in the KMnO4 oxidation). There may be more than one correct answer, and chemically correct steps will be accepted. Extra points will be given if correct names are provided. The points earned here will be applied to your lowest exam score! H Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY