
To review:
The chemical structures that are involved in the ionization of acetic acid in water.
Introduction:
The water is a polar and universal solvent. Most of the molecules having ionic bonds dissolve in water by making bonds with it. The compound dissociates into the individual ions making the ionic bond weaker than the solid form of the compound.
Explanation of Solution
The acetoacetic acid dissociates into the acetoacetate
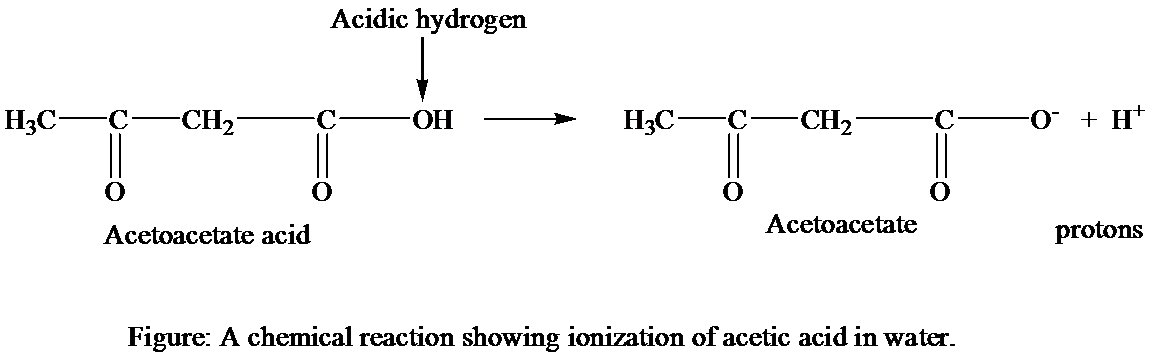
Thus, it can be concluded that the acetoacetate and hydrogen ions are chemical structures that are involved in the ionization of acetoacetic acid in water. The carboxyl group of acetoacetate infers the negative charge on the molecule and hydrogen ion gets dissociated from acetoacetic acid with its positive charge.
Want to see more full solutions like this?
Chapter 2 Solutions
LIFE:SCIENCE OF BIOL.(LL) >CUSTOM<
- Your goal is to produce black seeds resistant to mold. So you make the same cross again (between a homozygous black seeded, mold susceptible parent and a homozygous white seeded and mold resistant parent), and, again, advance progeny by SSD to create 100 F10 generation plants. Based on the information you obtained from your first crossing experiment (Question #4), how many F10 plants would you expect to have black seeds and be resistant to mold? Assume that a toxin produced by the mold fungus has been isolated. Only mold resistant seeds will germinate in the presence of the toxin. Could you use this toxin screening procedure to have segregation distortion work in your favor in the F2 generation? Explain your answer. Info from Question 4 a. P Locus (Seed Color): Hypothesis: The null hypothesis (H₀) is that seed color is controlled by alleles at a single locus. Observed Data: Total white seeds: 45 (resistant plants) + 6 (susceptible plants) = 51 Total black seeds: 7 (resistant…arrow_forward10. Consider the following enzyme and its substrate where the "+" and "-" indicate cations and anions, respectively. Explain which of the following inhibitors could inhibit this enzyme? Which type of inhibitor would it be and why? (Video 5-2) Substrate Enzyme Potential inhibitorsarrow_forwardUsing Punnett Squares Punnett squares are one good way to predict the outcome of genetic crosses. Punnett squares use mathematical probability to help predict the genotype and phenotype combinations in genetic crosses. The number of possible alleles from each parent determines the number of rows and columns in the Punnett square. Independent Assortment KEY QUESTION How do alleles segregate when more than one gene is involved? Mendel wondered if the segregation of one pair of alleles affects another pair. For example, does the gene that determines the shape of a seed affect the gene for seed color? This type of experiment is known as a two-factor, or dihybrid, cross because it involves two different genes. Single-gene crosses are monohybrid crosses. Visual Reading Tool: Two-Factor Cross: F₂ The Punnett square shows the results of self-crossing the F, generation of a cross between round yellow peas and wrinkled green peas. 1. List the different genotypes in the F, generation. What is the…arrow_forward
- CHAPTER 12 LESSON 2 Applying Mendel's Principles READING TOOL Connect to Visuals Before you read, preview Figure 12-7. Try to infer the purpose of this diagram. As you read, compare your inference to the text. After you read, revise your statement if needed or write a new one about the diagram's purpose. Take notes on the lines provided. Then view the Punnett square and answer the questions below regarding the genotypes and phenotypes. Inference: Revision: Parent 2 rryy Gametes F ry Parent 1 RRYY Gametes RY RrYy The F, generation are all RrYy. 1. What is the phenotype of parent 1?. 2. What is the genotype of parent 1? 3. What is the phenotype of parent 2? 4. What is the genotype of parent 2? 5. What is the phenotype of the F, offspring?. 6. What is the genotype of the F, offspring?. 7. What kind of cross does this figure describe? 144 Chanter 12 Introduction to Genetice Copyright Pearson Education Inc. or its affiliator. All rights reserved.arrow_forwardHow is the term enzyme related to the term proteinarrow_forwardCan very low temperatures cause proteins to denature? Explain why or why not?arrow_forward
- Humans consider themselves amazingly clever and innovative, constantly developing "new" ways of altering the world around us. As material consumption has increased, many have turned to the ideas of recycling and reuse as a means to minimize some negative aspects of our modern consumerism. Mother Nature though is the ultimate innovator and, more importantly, the ultimate recycler.arrow_forwardH gene assorts independently from the I gene. Both on autosomes. One man and one woman, both of HhIAIB genotype. Determine the blood type of progeny and fractions out of 16arrow_forwardAlleles at the P locus control seed color. Plants which are pp have white seeds, white flowers and no pigment in vegetative parts. Plants which are P_ have black seeds, purple flowers and may have varying degrees of pigment on stems and leaves. Seed color can be assessed, visually, based on if the seed is white or not white A gene for mold resistance has been reported and we want to determine its inheritance and whether it is linked to P. For the purposes of this exercise, we will assume that resistance is controlled by a single locus M, and M_ plants are resistant and mm plants are susceptible. Resistance can be measured, under greenhouse conditions, 2 weeks after planting, by injecting each seedling with a spore suspension. After two weeks, the seedlings can be rated as resistant or susceptible, based on whether or not tissue is actively sporulating. For this exercise we will use seed and data from the F10 generation of a recombinant inbred population produced using single seed…arrow_forward
- Linkage in common bean Alleles at the P locus control seed color. Plants which are pp have white seeds, white flowers and no pigment in vegetative parts. Plants which are P_ have black seeds, purple flowers and may have varying degrees of pigment on stems and leaves. Seed color can be assessed, visually, based on if the seed is white or not white A gene for mold resistance has been reported and we want to determine its inheritance and whether it is linked to P. For the purposes of this exercise, we will assume that resistance is controlled by a single locus M, and M_ plants are resistant and mm plants are susceptible. Resistance can be measured, under greenhouse conditions, 2 weeks after planting, by injecting each seedling with a spore suspension. After two weeks, the seedlings can be rated as resistant or susceptible, based on whether or not tissue is actively sporulating. For this exercise we will use seed and data from the F10 generation of a recombinant inbred population…arrow_forwardAlleles at the P locus control seed color. Plants which are pp have white seeds, white flowers and no pigment in vegetative parts. Plants which are P_ have black seeds, purple flowers and may have varying degrees of pigment on stems and leaves. Seed color can be assessed, visually, based on if the seed is white or not white A gene for mold resistance has been reported and we want to determine its inheritance and whether it is linked to P. For the purposes of this exercise, we will assume that resistance is controlled by a single locus M, and M_ plants are resistant and mm plants are susceptible. Resistance can be measured, under greenhouse conditions, 2 weeks after planting, by injecting each seedling with a spore suspension. After two weeks, the seedlings can be rated as resistant or susceptible, based on whether or not tissue is actively sporulating. For this exercise we will use seed and data from the F10 generation of a recombinant inbred population produced using single seed…arrow_forwardcan you help? I think its B but not surearrow_forward
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





