
Fill in the blanks in this concept map to help you tie together the key concepts concerning elements, atoms, and molecules.
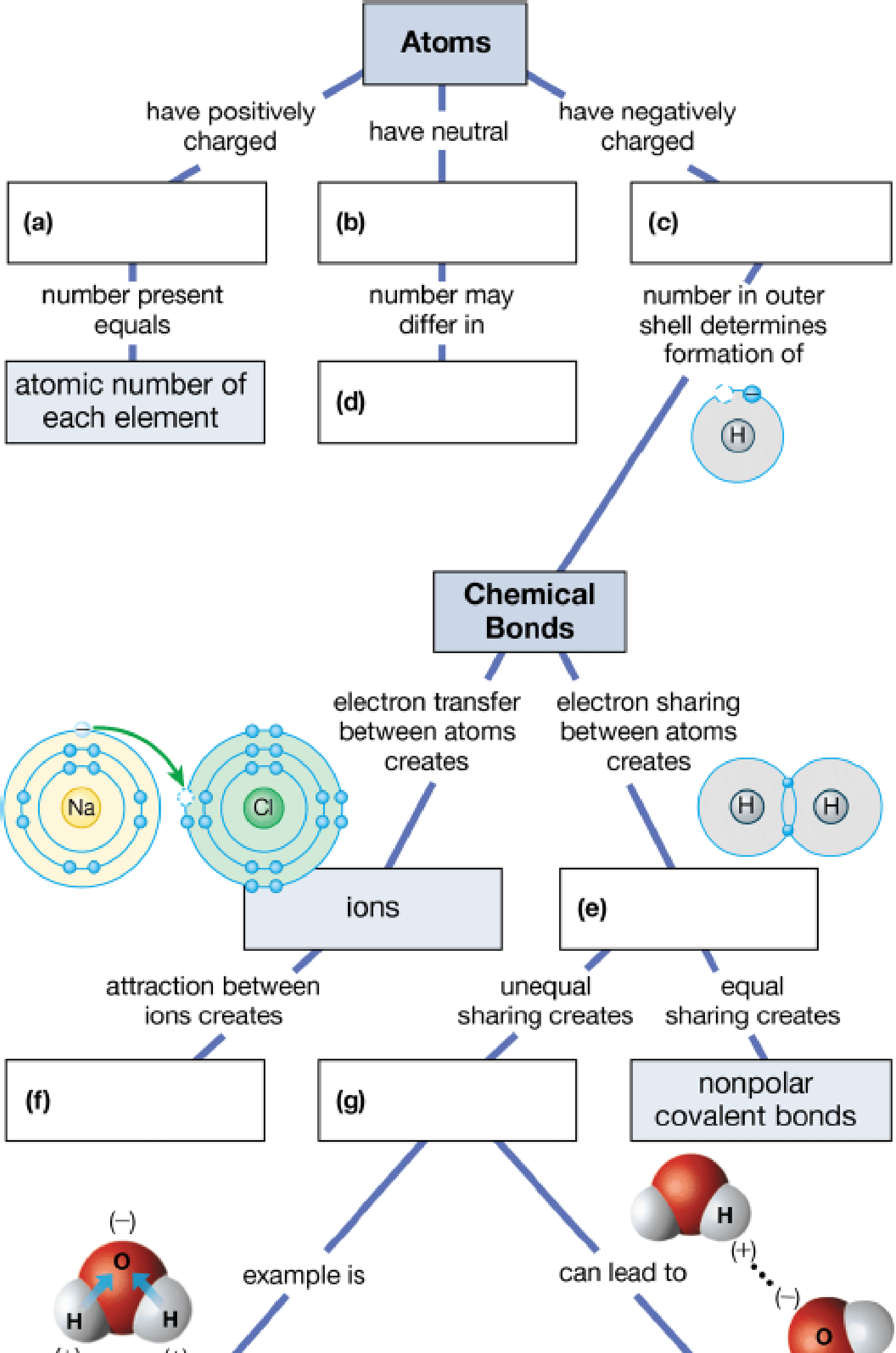

To complete: The concept map to help tie together the key concepts concerned with elements, atoms, and molecules.
Introduction:
A substance that cannot be broken down to other substances by ordinary chemical process is known as element. Every element has the smallest unit of matter that retains the properties of an element. This smallest unit is called atom. An atom consists of electrons (e), protons (p) and neutrons (n). Different atoms have specific numbers of electrons, protons and neutrons. Nucleus of an atom contains protons and neutrons that constitute the mass of an atom. Electrons revolve around the nucleus in their orbit. The atomic number of an element is the number of protons in the nucleus of its atoms.
Answer to Problem 1CC
Pictorial representation: The Fig. 1 shows a concept map for hierarchy from atoms to bonding between molecules.
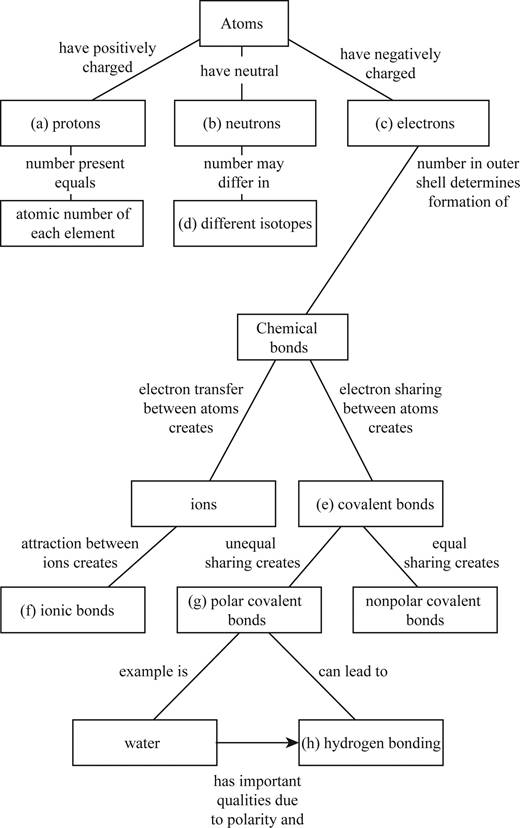
Fig.1:Concepts map of elements, atoms and molecules.
Explanation of Solution
(a)
Correct answer: Protons
Explanation: Proton is present in the nucleus of the atom and has positive charge. Hence the correct answer is proton.
(b)
Correct answer: Neutrons
Explanation: Neutron is present in the nucleus of the atom and is neutral due to no charge.
Hence, the correct answer is neutrons.
(c)
Correct answer: Electrons
Explanation: Electron revolves around the nucleus and has negative charge.
Hence, the correct answer is electrons.
(d)
Correct answer: Different isotopes
Explanation:
An isotope of an atom differs from neutron numbers and therefore by mass. Hence, the correct answer is different isotopes.
(e)
Correct answer: Covalent bond
Explanation: Electrons revolve around the nucleus in different electronic shells. The electrons present in the outermost shell are called valence electrons. The valence electron takes part in chemical bond formation by electron sharing known as covalent bond.
Hence, the correct answer is covalent bond.
(f)
Correct answer: Ionic bonds
Explanation: The transfer of electrons between two atoms creates ions and its attraction creates ionic bond. Hence, the correct answer is ionic bonds.
(g)
Correct answer: Polar covalent bonds
Explanation: Electron sharing between atoms creates covalent bond. The more electronegative atom attracts the bonded electrons towards itself. This results in unequal sharing of electrons and formation of polar covalent bond.
Hence, the correct answer is polar covalent bonds.
(h)
Correct answer: Hydrogen bonding
Explanation: Unequal sharing of electrons of covalent bond results in polar covalent bond. For example: Water molecule (H2O) consists of two hydrogen atoms and one oxygen atom. Oxygen atom shares one electron to each of the hydrogen atom and forms two covalent bonds. The oxygen is more electronegative than hydrogen. It attracts the bonded electron towards itself. This induces partial positive charge on hydrogen atoms and partial negative charge on oxygen atom. So, water molecule attains polarity. Oxygen atom of water molecule form a weak bond called hydrogen bond with hydrogen atom of another water molecule. This makes water molecule to have unique qualities.
Hence, the correct answer is hydrogen bonding.
Want to see more full solutions like this?
Chapter 2 Solutions
Campbell Biology Concepts and Connections, Third custom edition for Orange Coast College, Includes Online Access code.
- Describe the levels of structural hierarchy for the human body, starting with the organismal level and ending with the chemical level. In addition, you should make sure you link each level to the previous level, emphasizing the structural relationships.arrow_forward9 S es Read the section "Investigating Life: In (Extremely) Cold Blood." Then, drag and drop the terms on the left to complete the concept map. Red blood cells Genes Icefishes -have mutated have colorless Oxygen have few lack encode Blood Cellular respiration consists of- contain carries is a Platelets White blood cells carries low amounts of Hemoglobin is necessary for Plasma Protein Reset.arrow_forwardPlating 50 microliters of a sample diluted by a factor of 10-6 produced 91 colonies. What was the originalcell density (CFU/ml) in the sample?arrow_forward
- Every tutor here has got this wrong, don't copy off them.arrow_forwardSuppose that the population from question #1 (data is in table below) is experiencing inbreeding depression (F=.25) (and no longer experiencing natural selection). Calculate the new expected genotype frequencies (f) in this population after one round of inbreeding. Please round to 3 decimal places. Genotype Adh Adh Number of Flies 595 Adh Adh 310 Adhs Adhs 95 Total 1000 fladh Adh- flAdn Adh fAdhs Adharrow_forwardWhich of the following best describes why it is difficult to develop antiviral drugs? Explain why. A. antiviral drugs are very difficult to develop andhave no side effects B. viruses are difficult to target because they usethe host cell’s enzymes and ribosomes tometabolize and replicate C. viruses are too small to be targeted by drugs D. viral infections usually clear up on their ownwith no problemsarrow_forward
- This question has 3 parts (A, B, & C), and is under the subject of Nutrition. Thank you!arrow_forwardThey got this question wrong the 2 previous times I uploaded it here, please make sure it's correvct this time.arrow_forwardThis question has multiple parts (A, B & C), and under the subject of Nutrition. Thank you!arrow_forward
- Calculate the CFU/ml of a urine sample if 138 E. coli colonies were counted on a Nutrient Agar Plate when0.5 mls were plated on the NA plate from a 10-9 dilution tube. You must highlight and express your answerin scientific notatioarrow_forwardDon't copy off the other answer if there is anyarrow_forwardAnswerarrow_forward

 Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





