
Fill in the blanks in this concept map to help you tie together the key concepts concerning elements, atoms, and molecules.
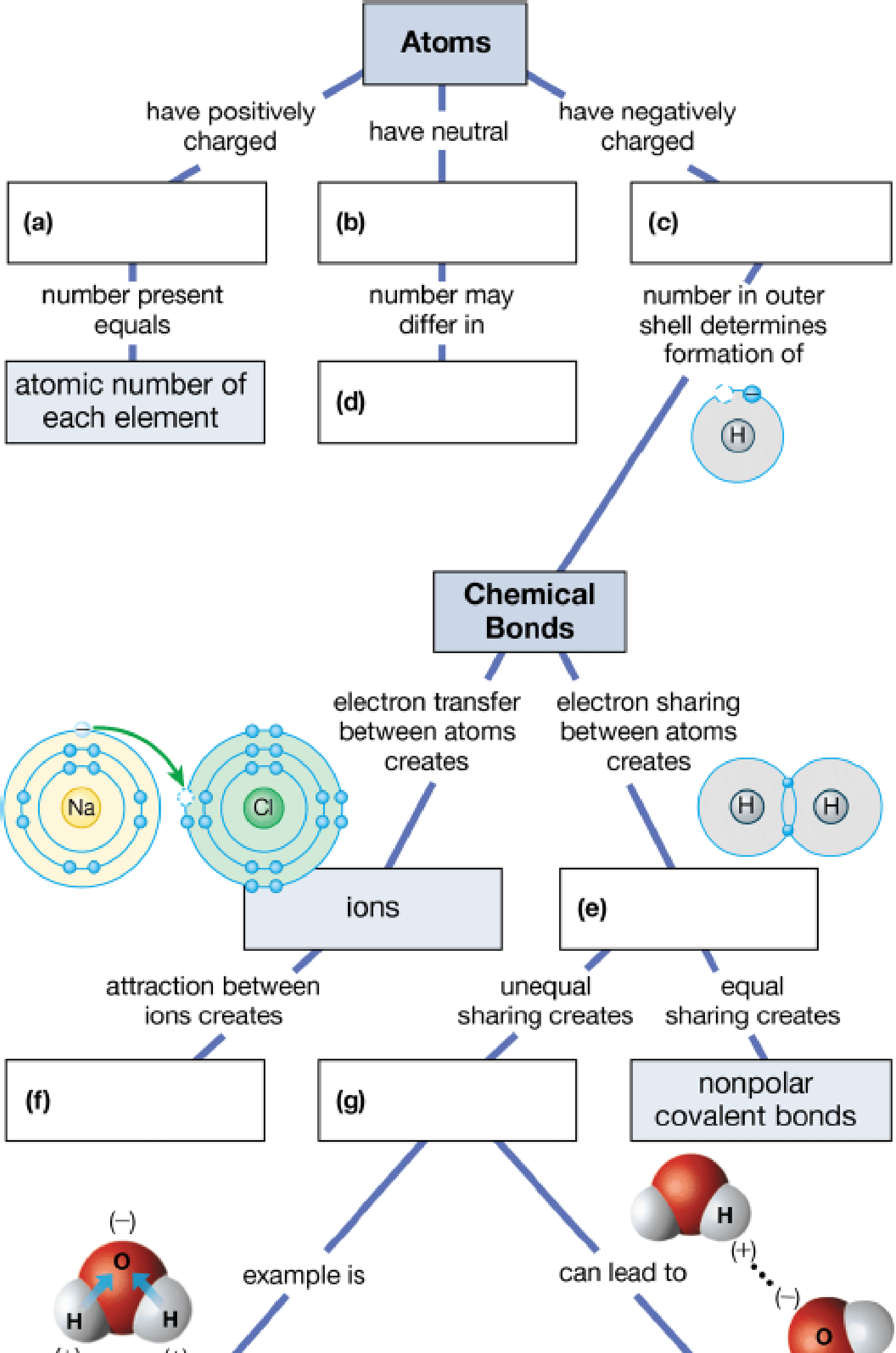

To complete: The concept map to help tie together the key concepts concerned with elements, atoms, and molecules.
Introduction:
A substance that cannot be broken down to other substances by ordinary chemical process is known as element. Every element has the smallest unit of matter that retains the properties of an element. This smallest unit is called atom. An atom consists of electrons (e), protons (p) and neutrons (n). Different atoms have specific numbers of electrons, protons and neutrons. Nucleus of an atom contains protons and neutrons that constitute the mass of an atom. Electrons revolve around the nucleus in their orbit. The atomic number of an element is the number of protons in the nucleus of its atoms.
Answer to Problem 1CC
Pictorial representation: The Fig. 1 shows a concept map for hierarchy from atoms to bonding between molecules.
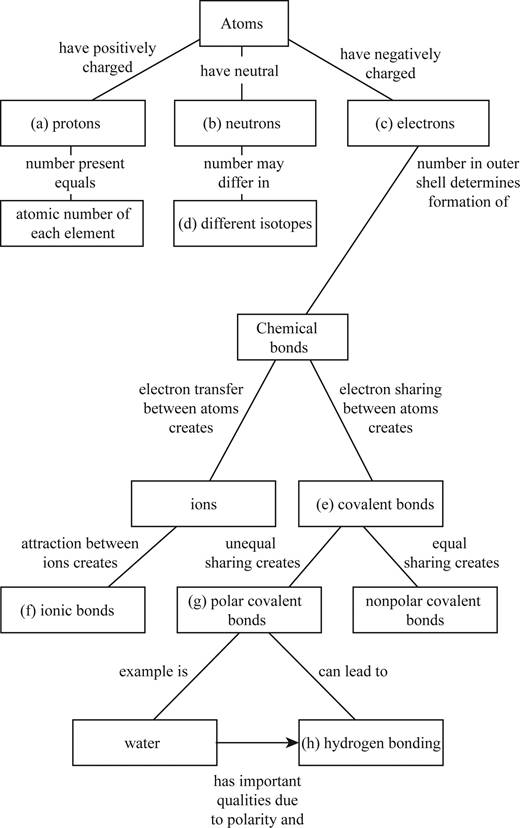
Fig.1:Concepts map of elements, atoms and molecules.
Explanation of Solution
(a)
Correct answer: Protons
Explanation: Proton is present in the nucleus of the atom and has positive charge. Hence the correct answer is proton.
(b)
Correct answer: Neutrons
Explanation: Neutron is present in the nucleus of the atom and is neutral due to no charge.
Hence, the correct answer is neutrons.
(c)
Correct answer: Electrons
Explanation: Electron revolves around the nucleus and has negative charge.
Hence, the correct answer is electrons.
(d)
Correct answer: Different isotopes
Explanation:
An isotope of an atom differs from neutron numbers and therefore by mass. Hence, the correct answer is different isotopes.
(e)
Correct answer: Covalent bond
Explanation: Electrons revolve around the nucleus in different electronic shells. The electrons present in the outermost shell are called valence electrons. The valence electron takes part in chemical bond formation by electron sharing known as covalent bond.
Hence, the correct answer is covalent bond.
(f)
Correct answer: Ionic bonds
Explanation: The transfer of electrons between two atoms creates ions and its attraction creates ionic bond. Hence, the correct answer is ionic bonds.
(g)
Correct answer: Polar covalent bonds
Explanation: Electron sharing between atoms creates covalent bond. The more electronegative atom attracts the bonded electrons towards itself. This results in unequal sharing of electrons and formation of polar covalent bond.
Hence, the correct answer is polar covalent bonds.
(h)
Correct answer: Hydrogen bonding
Explanation: Unequal sharing of electrons of covalent bond results in polar covalent bond. For example: Water molecule (H2O) consists of two hydrogen atoms and one oxygen atom. Oxygen atom shares one electron to each of the hydrogen atom and forms two covalent bonds. The oxygen is more electronegative than hydrogen. It attracts the bonded electron towards itself. This induces partial positive charge on hydrogen atoms and partial negative charge on oxygen atom. So, water molecule attains polarity. Oxygen atom of water molecule form a weak bond called hydrogen bond with hydrogen atom of another water molecule. This makes water molecule to have unique qualities.
Hence, the correct answer is hydrogen bonding.
Want to see more full solutions like this?
Chapter 2 Solutions
Campbell Biology: Concepts & Connections (8th Edition)
- 1.Steroids like testosterone and estrogen are nonpolar and large (~18 carbons). Steroids diffuse through membranes without transporters. Compare and contrast the remaining substances and circle the three substances that can diffuse through a membrane the fastest, without a transporter. Put a square around the other substance that can also diffuse through a membrane (1000x slower but also without a transporter). Molecule Steroid H+ CO₂ Glucose (C6H12O6) H₂O Na+ N₂ Size (Small/Big) Big Nonpolar/Polar/ Nonpolar lonizedarrow_forwardwhat are the answer from the bookarrow_forwardwhat is lung cancer why plants removes liquid water intead water vapoursarrow_forward
- *Example 2: Tracing the path of an autosomal dominant trait Trait: Neurofibromatosis Forms of the trait: The dominant form is neurofibromatosis, caused by the production of an abnormal form of the protein neurofibromin. Affected individuals show spots of abnormal skin pigmentation and non-cancerous tumors that can interfere with the nervous system and cause blindness. Some tumors can convert to a cancerous form. i The recessive form is a normal protein - in other words, no neurofibromatosis.moovi A typical pedigree for a family that carries neurofibromatosis is shown below. Note that carriers are not indicated with half-colored shapes in this chart. Use the letter "N" to indicate the dominant neurofibromatosis allele, and the letter "n" for the normal allele. Nn nn nn 2 nn Nn A 3 N-arrow_forwardI want to be a super nutrition guy what u guys like recommend mearrow_forwardPlease finish the chart at the bottom. Some of the answers have been filled in.arrow_forward
- 9. Aerobic respiration of one lipid molecule. The lipid is composed of one glycerol molecule connected to two fatty acid tails. One fatty acid is 12 carbons long and the other fatty acid is 18 carbons long in the figure below. Use the information below to determine how much ATP will be produced from the glycerol part of the lipid. Then, in part B, determine how much ATP is produced from the 2 fatty acids of the lipid. Finally put the NADH and ATP yields together from the glycerol and fatty acids (part A and B) to determine your total number of ATP produced per lipid. Assume no other carbon source is available. 18 carbons fatty acids 12 carbons 9 glycerol A. Glycerol is broken down to glyceraldehyde 3-phosphate, a glycolysis intermediate via the following pathway shown in the figure below. Notice this process costs one ATP but generates one FADH2. Continue generating ATP with glyceraldehyde-3-phosphate using the standard pathway and aerobic respiration. glycerol glycerol-3- phosphate…arrow_forwardNormal dive (for diving humans) normal breathing dive normal breathing Oz level CO2 level urgent need to breathe Oz blackout zone high CO2 triggers breathing 6. This diagram shows rates of oxygen depletion and carbon dioxide accumulation in the blood in relation to the levels needed to maintain consciousness and trigger the urgent need to breathe in diving humans. How might the location and slope of the O₂ line differ for diving marine mammals such as whales and dolphins? • How might the location and slope of the CO₂ line differ for diving marine mammals such as whales and dolphins? • • Draw in predicted lines for O2 and CO2, based on your reasoning above. How might the location of the Urgent Need to Breathe line and the O2 Blackout Zone line differ for diving marine mammals? What physiological mechanisms account for each of these differences, resulting in the ability of marine mammals to stay submerged for long periods of time?arrow_forwardforaging/diet type teeth tongue stomach intestines cecum Insectivory numerous, spiky, incisors procumbentExample: moleExample: shrew -- simple short mostly lacking Myrmecophagy absent or reduced in numbers, peg-likeExample: tamandua anteater extremely long simple, often roughened short small or lacking Terrestrial carnivory sharp incisors; long, conical canines; often carnassial cheek teeth; may have crushing molarsExample: dog -- simple short small Aquatic carnivory homodont, spiky, numerousExample: common dolphin -- simple or multichambered (cetaceans only) variable small or absent Sanguinivory very sharp upper incisors; reduced cheek teethExample: vampire bat grooved tubular, highly extensible long small or lacking Herbivory (except nectivores) incisors robust or absent; canines reduced or absent; diastema; cheek teeth enlarged with complex occlusal surfacesExample: beaver -- simple (hindgut fermenters) or multichambered (ruminants) long large Filter feeding none…arrow_forward
- 3. Shown below is the dental formula and digestive tract anatomy of three mammalian species (A, B, and C). What kind of diet would you expect each species to have? Support your answers with what you can infer from the dental formula and what you can see in the diagram. Broadly speaking, what accounts for the differences? Species A 3/3, 1/1, 4/4, 3/3 པར『ན་ cm 30 Species B 4/3, 1/1, 2/2, 4/4 cm 10 Species C 0/4, 0/0,3/3, 3/3 020arrow_forward3. Shown below is the dental formula and digestive tract anatomy of three mammalian species (A, B, and C). What kind of diet would you expect each species to have? Support your answers with what you can infer from the dental formula and what you can see in the diagram. Broadly speaking, what accounts for the differences? Species A 3/3, 1/1, 4/4, 3/3 cm 30 Species B 0/4, 0/0, 3/3, 3/3 cm 10 Species C 4/3, 1/1, 2/2, 4/4 E 0 cm 20 AILarrow_forwardNormal dive (for diving humans) normal breathing dive normal breathing Oz level CO₂ level urgent need to breathe Oz blackout zone high CO₂ triggers breathing 6. This diagram shows rates of oxygen depletion and carbon dioxide accumulation in the blood in relation to the levels needed to maintain consciousness and trigger the urgent need to breathe in diving humans. • How might the location and slope of the O2 line differ for diving marine mammals such as whales and dolphins? • How might the location and slope of the CO2 line differ for diving marine mammals such as whales and dolphins? • • Draw in predicted lines for O2 and CO2, based on your reasoning above. How might the location of the Urgent Need to Breathe line and the O2 Blackout Zone line differ for diving marine mammals? What physiological mechanisms account for each of these differences, resulting in the ability of marine mammals to stay submerged for long periods of time?arrow_forward

 Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





