
Fill in the blanks in this concept map to help you tie together the key concepts concerning elements, atoms, and molecules.
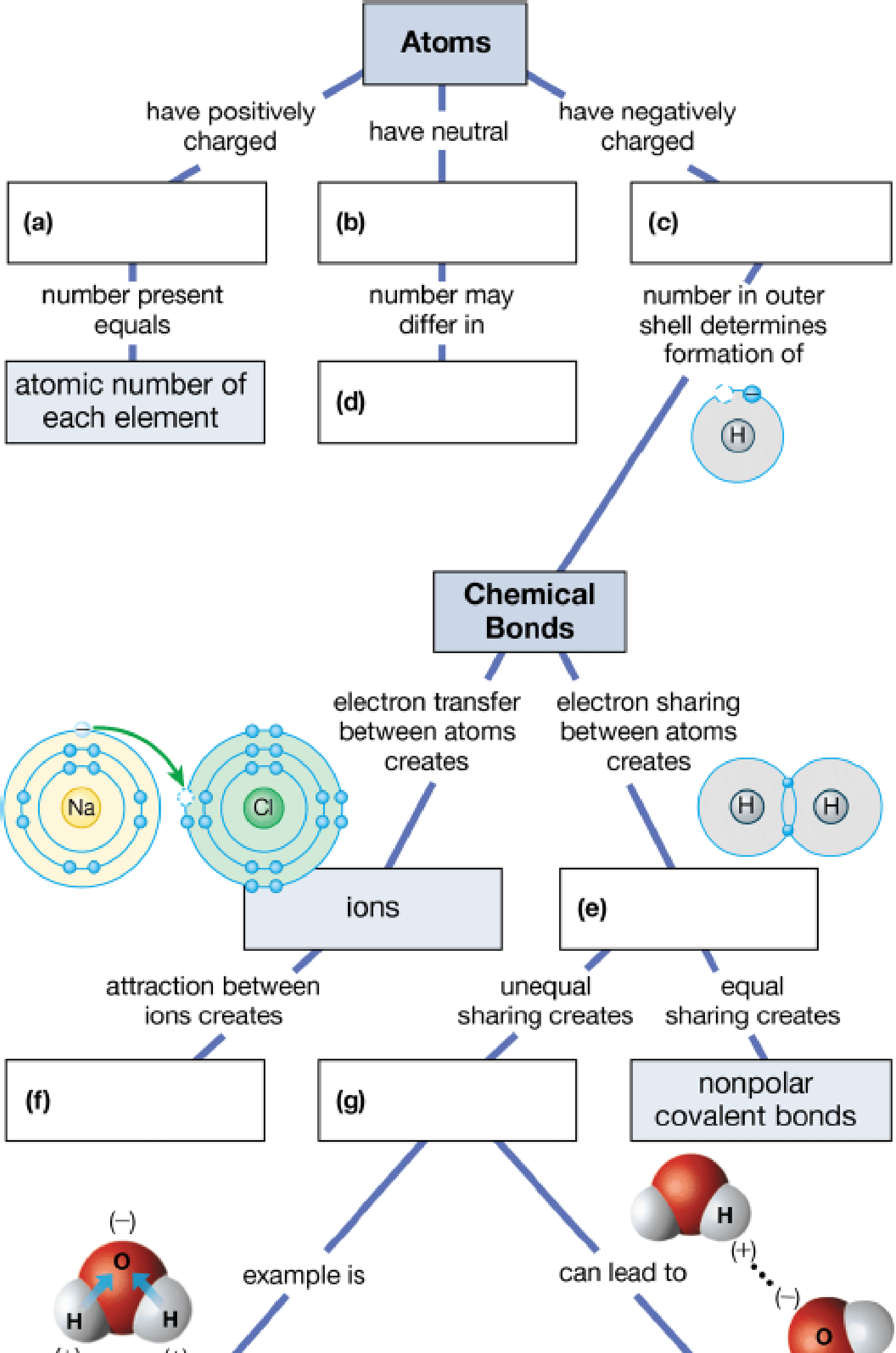

To complete: The concept map to help tie together the key concepts concerned with elements, atoms, and molecules.
Introduction:
A substance that cannot be broken down to other substances by ordinary chemical process is known as element. Every element has the smallest unit of matter that retains the properties of an element. This smallest unit is called atom. An atom consists of electrons (e), protons (p) and neutrons (n). Different atoms have specific numbers of electrons, protons and neutrons. Nucleus of an atom contains protons and neutrons that constitute the mass of an atom. Electrons revolve around the nucleus in their orbit. The atomic number of an element is the number of protons in the nucleus of its atoms.
Answer to Problem 1CC
Pictorial representation: The Fig. 1 shows a concept map for hierarchy from atoms to bonding between molecules.
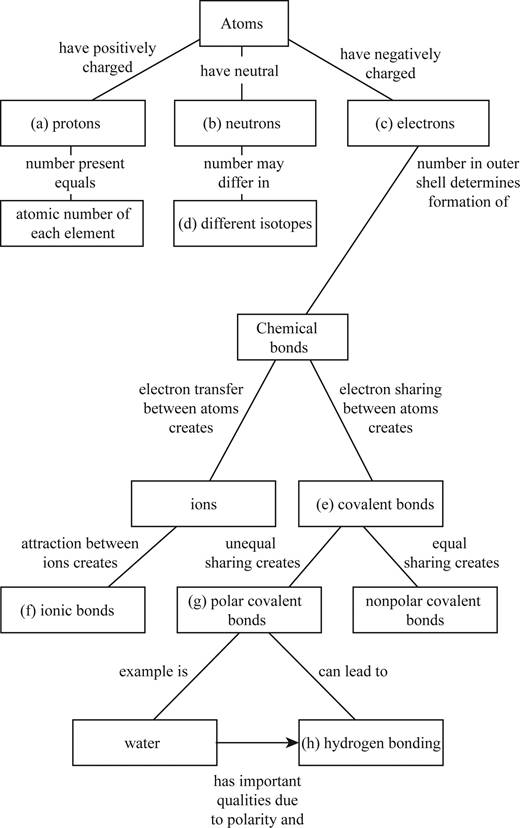
Fig.1:Concepts map of elements, atoms and molecules.
Explanation of Solution
(a)
Correct answer: Protons
Explanation: Proton is present in the nucleus of the atom and has positive charge. Hence the correct answer is proton.
(b)
Correct answer: Neutrons
Explanation: Neutron is present in the nucleus of the atom and is neutral due to no charge.
Hence, the correct answer is neutrons.
(c)
Correct answer: Electrons
Explanation: Electron revolves around the nucleus and has negative charge.
Hence, the correct answer is electrons.
(d)
Correct answer: Different isotopes
Explanation:
An isotope of an atom differs from neutron numbers and therefore by mass. Hence, the correct answer is different isotopes.
(e)
Correct answer: Covalent bond
Explanation: Electrons revolve around the nucleus in different electronic shells. The electrons present in the outermost shell are called valence electrons. The valence electron takes part in chemical bond formation by electron sharing known as covalent bond.
Hence, the correct answer is covalent bond.
(f)
Correct answer: Ionic bonds
Explanation: The transfer of electrons between two atoms creates ions and its attraction creates ionic bond. Hence, the correct answer is ionic bonds.
(g)
Correct answer: Polar covalent bonds
Explanation: Electron sharing between atoms creates covalent bond. The more electronegative atom attracts the bonded electrons towards itself. This results in unequal sharing of electrons and formation of polar covalent bond.
Hence, the correct answer is polar covalent bonds.
(h)
Correct answer: Hydrogen bonding
Explanation: Unequal sharing of electrons of covalent bond results in polar covalent bond. For example: Water molecule (H2O) consists of two hydrogen atoms and one oxygen atom. Oxygen atom shares one electron to each of the hydrogen atom and forms two covalent bonds. The oxygen is more electronegative than hydrogen. It attracts the bonded electron towards itself. This induces partial positive charge on hydrogen atoms and partial negative charge on oxygen atom. So, water molecule attains polarity. Oxygen atom of water molecule form a weak bond called hydrogen bond with hydrogen atom of another water molecule. This makes water molecule to have unique qualities.
Hence, the correct answer is hydrogen bonding.
Want to see more full solutions like this?
Chapter 2 Solutions
Campbell Biology: Concepts and Connections
- Consider the figure below. Suppose the true price of a health care service is P1. Suppose further that the individual has obtained insurance that has a fixed copayment for this particular service. The copayment is represented by price P2. represents the quantity of the service the individual would consume without insurance. quantity of the service the individual would consume with the insurance. Health Care Service represents the P. P₂ a Q1;Q2 Q2; Q3 Q1; Q3 Q3; Q1 Q2; Q1 फ f Q ८ g d h Q3\D 7Q 00000arrow_forwardThe table shows the utility Jordan receives at various income levels, but they do not know what their income will be next year. There is a 15% chance their income will be $25,000, a 20% chance their income will be $35,000, and a 65% chance their income will be $45,000. We know that Jordan is Income $25,000 Utility 2,800 30,000 3,200 35,000 3,500 40,000 3,700 45,000 3,800 ☐ none of the above 0 000 risk taker (lover) because their marginal utility of income is increasing risk neutral because their marginal utility of income is constant risk averse because their marginal utility of income is decreasing risk neutral because their marginal utility of income is decreasingarrow_forwardOOOO a d+e d a+b+c Consider the figure below. Suppose the true price of a health care service is P1. Suppose further that the individual has obtained insurance that has a fixed copayment for this particular service. The copayment is represented by price P2. The social loss from moral hazard if the individual has copayment P2 is represented graphically by the area(s): Health Care Service P. a No 4 ८ e g Q2 Q3 Darrow_forward
- OOO O The table shows the utility Jordan receives at various income levels, but they do not know what their income will be next year. There is a 15% chance their income will be $25,000, a 20% chance their income will be $35,000, and a 65% chance their income will be $45,000. We know that Jordan's expected income is. Their utility from their expected income is_ Income $25,000 Utility 2,800 30,000 3,200 35,000 3,500 40,000 3,700 45,000 3,800 $45,000; 3,800 $40,000; 3,700 $25,000; 2,800 $35,000; 3,500 $30,000; 3,200arrow_forwardQuestion 1 Classify the Bird Mark 7; how is it: Powered Triggered Cycled Classify brid mark 7 Powered: By gas (oxygen) Triggered: Negative Pressure, caused by the patient’s inspiratory effort Cycled: The machine stops delivering gas and allows for exhalationarrow_forwardHypothetical "pedigree" for Sickle Cellarrow_forward
- would this be considered a novel protein and if not how can I fix it so it is and can you draw the corrections pleasearrow_forwardIn as much detail as possible, hand draw a schematic diagram of the hypothalamic-pituitary- gonad (HPG) axis in the human male. Be sure to include all the relevant structures and hormones. You must define all abbreviations the first time you use them. Please include (and explain) the feedback loops.arrow_forwardA negligence action was brought by a mother against a hospital on behalf of her minor daughter. It alleged that when the mother was 13 years of age, the hospital negligently transfused her with Rh-positive blood. The mother's Rh-negative blood was incompatible with and sensitized by the Rh-positive blood. The mother discovered her condition 8 years later during a routine blood screening ordered by her healthcare provider in the course of prenatal care. The resulting sensitization of the mother's blood allegedly caused damage to the fetus, resulting in physical defects and premature birth. Did a patient relationship with the transfusing hospital exist?arrow_forward
- 18. Watch this short youtube video about SARS CoV-2 replication. SARS-CoV-2 Life Cycle (Summer 2020) - YouTube.19. What is the name of the receptor that SARS CoV-2 uses to enter cells? Which human cells express this receptor? 20. Name a few of the proteins that the SARS CoV-2 mRNA codes for. 21. What is the role of the golgi apparatus related to SARS CoV-2arrow_forwardState the five functions of Globular Proteins, and give an example of a protein for each function.arrow_forwardDiagram of check cell under low power and high powerarrow_forward

 Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





