
Concept explainers
(a)
Interpretation:
The stronger acid has to be determined.
Concept Introduction:
Base and its conjugate Acid: A Base is a species that can gain a proton. When a base gains a proton
Electron delocalization stabilizes base: If a base has delocalized electrons, then the negative charge that results when the base’s conjugate acid loses a proton will belong to one atom. If a base has delocalized electrons, then the negative charge that results when the base’s conjugate acid loses a proton will be shared by two or more atoms. A base with delocalized electron is more stable than a similar base with localized electrons.
Relative Acid strength: Replacing hydrogen with an electronegative substituent that pulls bonding electrons toward itself increases the strength of the acid.
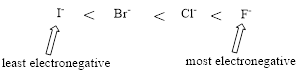
The more electronegative the substituent that replaces hydrogen, the stronger the acid becomes.
Example:
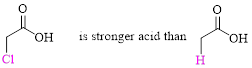
(b)
Interpretation:
The stronger acid has to be determined.
Concept Introduction:
Base and its conjugate Acid: A Base is a species that can gain a proton. When a base gains a proton
Electron delocalization stabilizes base: If a base has delocalized electrons, then the negative charge that results when the base’s conjugate acid loses a proton will belong to one atom. If a base has delocalized electrons, then the negative charge that results when the base’s conjugate acid loses a proton will be shared by two or more atoms. A base with delocalized electron is more stable than a similar base with localized electrons.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
- Briefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forwardTry: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
