
Concept explainers
a) Indole STRUCTURE GIVEN IN QUESTION
Interpretation:
The structure of indole is given. Its skeletal structure is to be drawn.
Concept introduction:
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. The end of a line represents a carbon atom with three hydrogen atoms, CH3; a two-way intersection is a carbon atom with two hydrogen atoms, CH2; a three way intersection is a carbon with one hydrogen, CH; a four way intersection is a carbon with no attached hydrogen. Atoms other than carbon and hydrogen are shown. Nitrogen is trivalent and it can form one double bond and a single bond or three single bonds.
To draw:
The skeletal structure of indole.
Answer to Problem 42AP
The skeletal structure of indole is
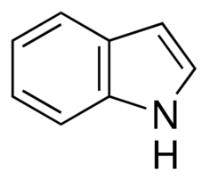
Explanation of Solution
The molecular formula of indole is C8H7N. It has bicyclic structure consisting of a six membered benzene ring fused to a five membered pyrrole ring. In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. A three way intersection is a carbon with one hydrogen, CH; a four way intersection is a carbon with no attached hydrogen. Atoms other than carbon and hydrogen are shown. Nitrogen is trivalent and it is in a three way intersection as NH.
The skeletal structure of indole is

b) 1,3- pentadiene
Interpretation:
The structure of 1,3- pentadiene is given. Its skeletal structure is to be drawn.
Concept introduction:
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. The end of a line represents a carbon atom with three hydrogen atoms, CH3; a two-way intersection is a carbon atom with two hydrogen atoms, CH2; a three way intersection is a carbon with one hydrogen, CH; a four way intersection is a carbon with no attached hydrogen. Atoms other than carbon and hydrogen are shown.
To draw:
The skeletal structure of 1,3- pentadiene.
Answer to Problem 42AP
The skeletal structure of 1,3- pentadiene is
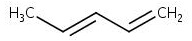
Explanation of Solution
The molecular formula of 1,3- pentadiene is C5H8. It has two double bonds, one between C1-C2 and another between C3-C4. In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. The end of a line represents a carbon atom with three hydrogen atoms, CH3; a two-way intersection is a carbon atom with two hydrogen atoms, CH2; a three way intersection is a carbon with one hydrogen, CH. Based on these concepts the structure of 1,3- pentadiene can be written.
The skeletal structure of 1,3- pentadiene is

c) 1,2- dichloro cyclopentane
Interpretation:
The structure of 1,2- dichloro cyclopentane is given. Its skeletal structure is to be drawn.
Concept introduction:
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. The end of a line represents a carbon atom with three hydrogen atoms, CH3; a two-way intersection is a carbon atom with two hydrogen atoms, CH2; a three way intersection is a carbon with one hydrogen, CH; a four way intersection is a carbon with no attached hydrogen. Atoms other than carbon and hydrogen are shown.
To draw:
The skeletal structure of 1,2- dichloro cyclopentane.
Answer to Problem 42AP
The skeletal structure of 1,2- dichloro cyclopentane is
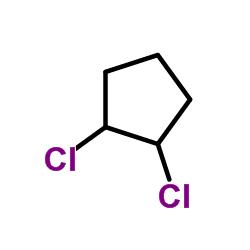
Explanation of Solution
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. Atoms other than carbon and hydrogen are shown. The molecular formula of 1,2- dichlorocyclopentene is C5H8Cl2. A cyclopentane ring is made up of five methylene groups. In 1,2- dichlorocyclopentene the two chlorine atoms are bonded to the first and second carbons replacing one hydrogen on each. They are shown in the skeletal structure.
The skeletal structure of 1,2- dichloro cyclopentane is

d) benzoquinone
Interpretation:
The structure of benzoquinone is given. Its skeletal structure is to be drawn.
Concept introduction:
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. Atoms other than carbon and hydrogen are shown.
To draw:
The skeletal structure of benzoquinone.
Answer to Problem 42AP
The skeletal structure of benzoquinone is
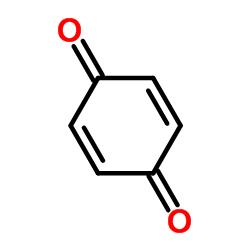
Explanation of Solution
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. Atoms other than carbon and hydrogen are shown. The molecular formula of benzoquinone is C6H4O2. The six carbons form a six membered ring with first and fourth carbons as carbonyl groups. The other four carbons have one hydrogen each.
The skeletal structure of benzoquinone is

Want to see more full solutions like this?
Chapter 1 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- Rank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forward
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



