
a)
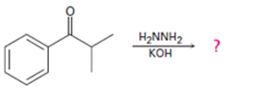
Interpretation:
The product of the Wolff-Kishner reduction reaction shown is to be given. The electron-pushing mechanism, beginning from the hydrazone intermediate is to be provided.
Concept introduction:
In Wolff-Kishner reduction
To give:
The product of the Wolff-Kishner reduction reaction shown.
To provide:
The electron-pushing mechanism, beginning from the hydrazone intermediate.
Answer to Problem 38MP
The product of the Wolff-Kishner reduction reaction shown is
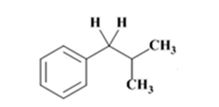
The electron-pushing mechanism for the formation of the alkane, beginning from the hydrazone intermediate, is given below.
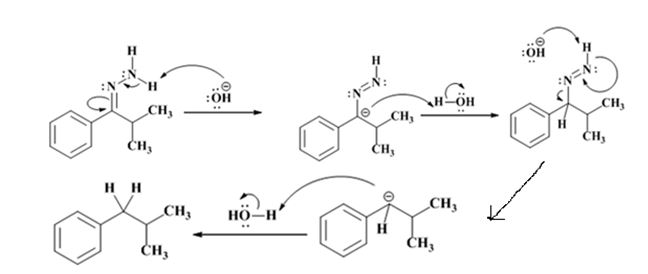
Explanation of Solution
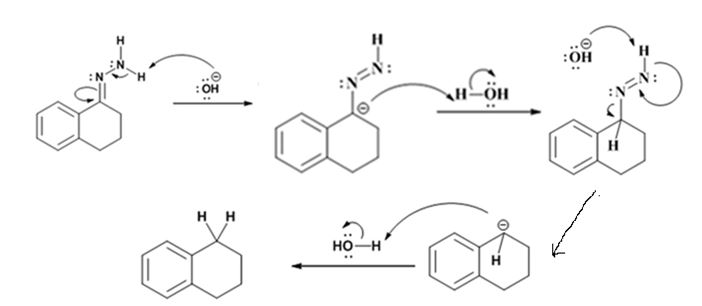
The hydroxide ion from KOH abstracts a weakly acidic H from –NH2 of the hydrazone of isopropyl phenyl ketone to yield a carbanion which picks up a proton to yield a neutral intermediate. Deprotonation of the remaining hydrogen on N by the hydroxide ion occurs with the eliminartion of nitrogen to yield another carbanion which is protonated to give the alkane, ethyl benzene, as the product.
The product of the Wolff-Kishner reduction reaction shown is

The electron-pushing mechanism, beginning from the hydrazone intermediate is given below.

b)
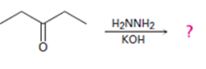
Interpretation:
The product of the Wolff-Kishner reduction reaction shown is to be given. The electron-pushing mechanism, beginning from the hydrazone intermediate is to be provided.
Concept introduction:
In Wolff-Kishner reduction aldehydes and ketones react with hydrazine in the presence of a base to yield alkanes. First a hydrazone is formed which is then converted into an alkane.
To give:
The product of the Wolff-Kishner reduction reaction shown.
To provide:
The electron-pushing mechanism the formation of the alkane beginning from the hydrazone intermediate.
Answer to Problem 38MP
The product of the Wolff-Kishner reduction reaction shown is
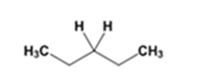
The electron-pushing mechanism the formation of the alkane, beginning from the hydrazone intermediate, is given below.
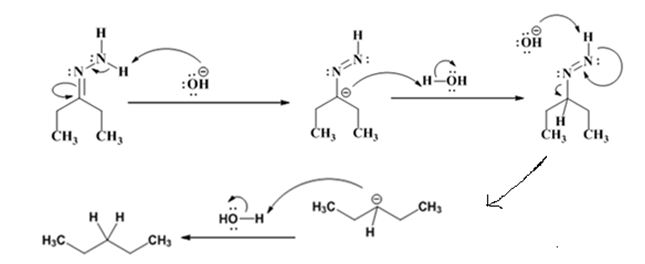
Explanation of Solution
The hydroxide ion from KOH abstracts a weakly acidic H from –NH2 of the hydrazone of diethyl ketone to yield a carbanion which picks up a proton to yield a neutral intermediate. Deprotonation of the remaining hydrogen on N by the hydroxide ion occurs with the eliminartion of nitrogen to yield another carbanion which is protonated to give the alkane, n-pentane, as the product.
The product of the Wolff-Kishner reduction reaction shown is

The electron-pushing mechanism, beginning from the hydrazone intermediate is given below.

c)
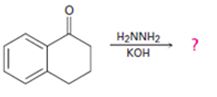
Interpretation:
The product of the Wolff-Kishner reduction reaction shown is to be given. The electron-pushing mechanism, beginning from the hydrazone intermediate is to be provided.
Concept introduction:
In Wolff-Kishner reduction aldehydes and ketones react with hydrazine in the presence of a base to yield alkanes. First a hydrazone is formed which is then converted into an alkane.
To give:
The product of the Wolff-Kishner reduction reaction shown.
To provide:
The electron-pushing mechanism the formation of the alkane beginning from the hydrazone intermediate.
Answer to Problem 38MP
The product of the Wolff-Kishner reduction reaction shown is
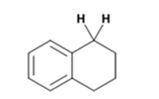
The electron-pushing mechanism the formation of the alkane, beginning from the hydrazone intermediate, is given below.
Explanation of Solution
The hydroxide ion from KOH abstracts a weakly acidic H from –NH2 of the hydrazone of the ketone to yield a carbanion which picks up a proton to yield a neutral intermediate. Deprotonation of the remaining hydrogen on N by the hydroxide ion occurs with the eliminartion of nitrogen to yield another carbanion which is protonated to give the alkane as the product.
The product of the Wolff-Kishner reduction reaction shown is

The electron-pushing mechanism, beginning from the hydrazone intermediate is given below.

d)
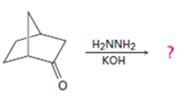
Interpretation:
The product of the Wolff-Kishner reduction reaction shown is to be given. The electron-pushing mechanism, beginning from the hydrazone intermediate is to be provided.
Concept introduction:
In Wolff-Kishner reduction aldehydes and ketones react with hydrazine in the presence of a base to yield alkanes. First a hydrazone is formed which is then converted into an alkane.
To give:
The product of the Wolff-Kishner reduction reaction shown.
To provide:
The electron-pushing mechanism, beginning from the hydrazone intermediate.
Answer to Problem 38MP
The product of the Wolff-Kishner reduction reaction shown is
The electron-pushing mechanism for the formation of the alkane beginning from the hydrazone intermediate, is given below.
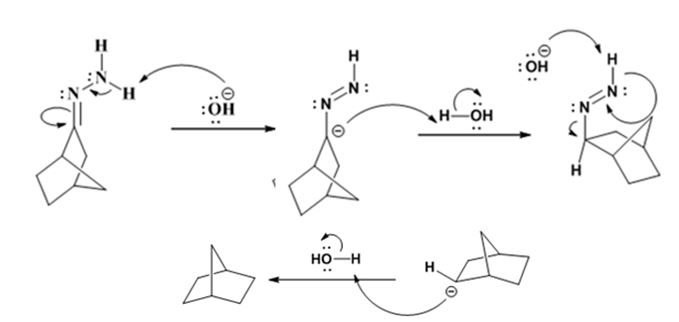
Explanation of Solution
The hydroxide ion from KOH abstracts a weakly acidic H from –NH2 of the hydrazone of ketone to yield a carbanion which picks up a proton to yield a neutral intermediate. Deprotonation of the remaining hydrogen on N by the hydroxide ion occurs with the eliminartion of nitrogen to yield another carbanion which is protonated to give the alkane as the product.
The product of the Wolff-Kishner reduction reaction shown is

The electron-pushing mechanism for the formation of the alkane beginning from the hydrazone intermediate, is given below.

Want to see more full solutions like this?
Chapter 19 Solutions
ORGANIC CHEMISTRY W/OWL
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


