
(a)
Interpretation: Synthesis of the given compound has to be proposed.
Concept Introduction:
Elimination Reaction: It is just reverse reaction of addition where substituent from the given molecule is removed.
E2 mechanism depends on both base and substituents in the reaction.
Elimination reaction of an
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
Hydroboration reaction: The reaction involves addition of
Oxidation: If electrons are moved from a species or oxygen atoms are added to a species or hydrogen atom gets removed from a species during a
Anti-Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the less substitution position of carbon-carbon double bond.
Wittig Reaction: It is an organic reaction where an
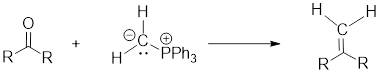
Chromic acid:
(b)
Interpretation: Synthesis of the given compound has to be proposed.
Concept Introduction:
Hydroboration reaction: The reaction involves addition of
Oxidation: If electrons are moved from a species or oxygen atoms are added to a species or hydrogen atom gets removed from a species during a chemical reaction is known as oxidation.
Anti-Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the less substitution position of carbon-carbon double bond.
Wittig Reaction: It is an organic reaction where an aldehyde or a ketone gets converted to an alkene by replacing carbonyl group by a
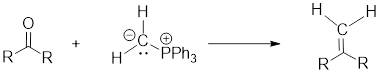
(c)
Interpretation: Synthesis of the given compound has to be proposed.
Concept Introduction:
Elimination Reaction: It is just reverse reaction of addition where substituent from the given molecule is removed.
E2 mechanism depends on both base and substituents in the reaction.
Elimination reaction of an alkyl halide results in the formation of an alkene.
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
Hydroboration reaction: The reaction involves addition of
Oxidation: If electrons are moved from a species or oxygen atoms are added to a species or hydrogen atom gets removed from a species during a chemical reaction is known as oxidation.
Anti-Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the less substitution position of carbon-carbon double bond.
Grignard Reaction: This is an organometallic reaction where an alkyl or aryl-magnesium halides is introduced to the carbonyl group present in an aldehyde and ketone. Here, aldehyde and ketone gets converted to alcohols.
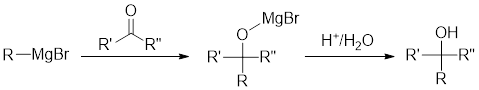
Chromic acid:
(d)
Interpretation: Synthesis of the given compound has to be proposed.
Concept Introduction:
Elimination Reaction: It is just reverse reaction of addition where substituent from the given molecule is removed.
E2 mechanism depends on both base and substituents in the reaction.
Elimination reaction of an alkyl halide results in the formation of an alkene.
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
Grignard Reaction: This is an organometallic reaction where an alkyl or aryl-magnesium halides is introduced to the carbonyl group present in an aldehyde and ketone. Here, aldehyde and ketone gets converted to alcohols.
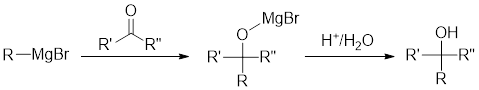
Chromic acid:
Ozonolysis: It is an organic reaction where the unsaturated bonds in alkenes and
(e)
Interpretation: Synthesis of the given compound has to be proposed.
Concept Introduction:
Hydroboration reaction: The reaction involves addition of
Oxidation: If electrons are moved from a species or oxygen atoms are added to a species or hydrogen atom gets removed from a species during a chemical reaction is known as oxidation.
Anti-Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the less substitution position of carbon-carbon double bond.
In a reaction, PCC (pyridinium chlorochromate) is used to oxidize alcohols to carbonyls. Primary alcohols get converted to aldehydes whereas secondary alcohols get converted to ketones when treated with PCC.
An imine is a compound having
The part of the molecule that is attached to the carbon atom in the
(f)
Interpretation: Synthesis of the given compound has to be proposed.
Concept Introduction:
Friedel-Crafts Acylation: This Lewis acid-catalyzed electrophilic aromatic substitution is the reaction between arenes and acyl chlorides or anhydrides for the synthesis of monoacylated compound. The products are deactivated, as well as do not undergo a second substitution.
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
Wittig Reaction: It is an organic reaction where an aldehyde or a ketone gets converted to an alkene by replacing carbonyl group by a
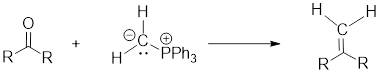
(g)
Interpretation: Synthesis of the given compound has to be proposed.
Concept Introduction:
An acetal is a compound having structural formula
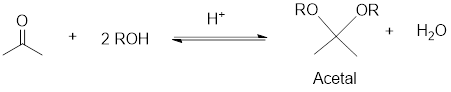
Reduction: If electrons are gained to a species or hydrogen atoms are added to a species or oxygen atom gets removed from a species during a chemical reaction is known as reduction
In a reaction,
Trending nowThis is a popular solution!

Chapter 19 Solutions
ORGANIC CHEMISTRY 3E WPNGC LL SET 1S
- Protecting Groups and Carbonyls 6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation, reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.) III + VI HS HS H+ CH,CH,Li III I II IV CI + P(Ph)3 V ༼ Hint: no strong base added VI S VII IX HO VIII -MgBr HgCl2,HgO HO. isomerization aqeuous solution H,SO, ༽༽༤༽༽ X MeOH Hint: enhances selectivity for reaction at the S X ☑arrow_forwardDraw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forward
- Show the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





