
PHYS 212 FOR SCI+ENG W/MAST PHYS >ICP<
1st Edition
ISBN: 9781323834831
Author: Knight
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 8CQ
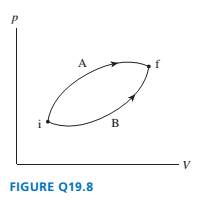
FIGURE Q19.8 shows two different processes taking an ideal gas
from state i to state f.
a. Is the temperature change ?T during process A larger than,
smaller than, or equal to the change during process B? Explain.
b. Is the heat energy added during process A greater than, less
than, or equal to the heat added during process B? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
According to a grade 11 Physics SPH3U course Kinematics, Dynamics, and Energy answer the following question
According to a grade 11 Physics SPH3U course Kinematics, Dynamics, and Energy answer the following question
According to a grade 11 Physics SPH3U course Kinematics, Dynamics, and Energy answer the following question
Chapter 19 Solutions
PHYS 212 FOR SCI+ENG W/MAST PHYS >ICP<
Ch. 19 - Prob. 1CQCh. 19 - Do (a) temperature, (b) heat, and (c) thermal...Ch. 19 - Prob. 3CQCh. 19 - You need to raise the temperature of a gas by...Ch. 19 - Prob. 5CQCh. 19 - Prob. 6CQCh. 19 - FIGURE Q19.7 shows two different processes taking...Ch. 19 - FIGURE Q19.8 shows two different processes taking...Ch. 19 - The gas cylinder in FIGURE Q19.9 is a rigid...Ch. 19 - The gas cylinder in FIGURE Q19.10 is well...
Ch. 19 - The gas cylinder in FIGURE Q19.11 is well...Ch. 19 - How much work is done on the gas in the process...Ch. 19 - Prob. 2EAPCh. 19 - Prob. 3EAPCh. 19 - A 2000 cm3 container holds 0.10 mol of helium gas...Ch. 19 - Prob. 5EAPCh. 19 - Prob. 6EAPCh. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - 9. Draw a first-law bar chart (see Figure 19.12)...Ch. 19 - Prob. 10EAPCh. 19 - J of work are done on a system in a process that...Ch. 19 - How much heat energy must be added to a...Ch. 19 - Prob. 13EAPCh. 19 - Prob. 14EAPCh. 19 - Prob. 15EAPCh. 19 - Prob. 16EAPCh. 19 - One way you keep from overheating is by...Ch. 19 - Prob. 18EAPCh. 19 - Two cars collide head-on while each is traveling...Ch. 19 - An experiment measures the temperature of a 500 g...Ch. 19 - 30 g of copper pellets are removed from a 300°C...Ch. 19 - A 750 g aluminum pan is removed from the stove and...Ch. 19 - A 50.0 g thermometer is used to measure the...Ch. 19 - A 500 g metal sphere is heated to 300°C, then...Ch. 19 - A 65 cm3 block of iron is removed from an 800°C...Ch. 19 - Prob. 26EAPCh. 19 - A container holds 1.0 g of oxygen at a pressure of...Ch. 19 - The volume of a gas is halved during an adiabatic...Ch. 19 - Prob. 29EAPCh. 19 - Prob. 30EAPCh. 19 - Prob. 31EAPCh. 19 - Prob. 32EAPCh. 19 - Prob. 33EAPCh. 19 - Prob. 34EAPCh. 19 - Prob. 35EAPCh. 19 - What maximum power can be radiated by a...Ch. 19 - Radiation from the head is a major source of heat...Ch. 19 - Prob. 38EAPCh. 19 - Prob. 39EAPCh. 19 - Prob. 40EAPCh. 19 - Prob. 41EAPCh. 19 - Prob. 42EAPCh. 19 - Prob. 43EAPCh. 19 - The specific heat of most solids is nearly...Ch. 19 - Prob. 45EAPCh. 19 - Prob. 46EAPCh. 19 - Prob. 47EAPCh. 19 - Prob. 48EAPCh. 19 - .0 mol of gas are at 30°C and a pressure of 1.5...Ch. 19 - A 6.0-cm-diameter cylinder of nitrogen gas has a...Ch. 19 - Prob. 51EAPCh. 19 - An ideal-gas process is described by p = cV 1/2 ,...Ch. 19 - Prob. 53EAPCh. 19 - Prob. 54EAPCh. 19 - Prob. 55EAPCh. 19 - Prob. 56EAPCh. 19 - Prob. 57EAPCh. 19 - .10 mol of nitrogen gas follow the two processes...Ch. 19 - Prob. 59EAPCh. 19 - Prob. 60EAPCh. 19 - Prob. 61EAPCh. 19 - Prob. 62EAPCh. 19 - Prob. 63EAPCh. 19 - Prob. 64EAPCh. 19 - Prob. 65EAPCh. 19 - Prob. 66EAPCh. 19 - Prob. 67EAPCh. 19 - Prob. 68EAPCh. 19 - Prob. 69EAPCh. 19 - A cylindrical copper rod and an iron rod with...Ch. 19 - Prob. 71EAPCh. 19 - Prob. 72EAPCh. 19 - Prob. 73EAPCh. 19 - Prob. 74EAPCh. 19 - Prob. 75EAPCh. 19 - Prob. 76EAPCh. 19 - Prob. 77EAPCh. 19 - Prob. 78EAPCh. 19 - Prob. 79EAPCh. 19 - Prob. 80EAPCh. 19 - Prob. 81EAPCh. 19 - Prob. 82EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Three point-like charges in the attached image are placed at the corners of an equilateral triangle as shown in the figure. Each side of the triangle has a length of 38.0 cm, and the point (C) is located half way between q1 and q3 along the side. Find the magnitude of the electric field at point (C). Let q1 = −2.80 µC, q2 = −3.40 µC, and q3 = −4.50 µC. Thank you.arrow_forwardThree point-like charges are placed as shown in the attach image, where r1 = r2 = 44.0 cm. Find the magnitude of the electric force exerted on the charge q3. Let q1 = -1.90 uC, q2 = -2.60 uC, and q3 = +3.60 uC. Thank you.arrow_forwardThe drawing attached shows an edge-on view of two planar surfaces that intersect and are mutually perpendicular. Surface (1) has an area of 1.90 m², while Surface (2) has an area of 3.90 m². The electric field in magnitude of 215 N/C. Find the magnitude of the electric flux through surface (1 and 2 combined) if the angle theta made between the electric field with surface (2) is 30.0 degrees. Thank you.arrow_forward
- A car driving at 27m/s veers to the left to avoid a deer in the road. The maneuver takes 2.0s and the direction of travel is altered by 20 degrees. What is the average acceleration during the constant speed maneuver? Do this in accordance with the example in the chapter.arrow_forwardNo No No Chatgpt pls will upvotearrow_forward2 C01: Physical Quantities, Units and Measurementscobris alinu zotinUD TRO Bendemeer Secondary School Secondary Three Express Physics Chpt 1: Physical Quantities, Unit and Measurements Assignment Name: Chen ShiMan loov neowled soria 25 ( 03 ) Class: 3 Respect 6 Date: 2025.01.22 1 Which group consists only of scalar quantities? ABCD A acceleration, moment and energy store distance, temperature and time length, velocity and current mass, force and speed B D. B Which diagram represents the resultant vector of P and Q? lehtele 시 bas siqpeq olarist of beau eldeo qirie-of-qi P A C -B qadmis rle mengaib priwollot erT S Quilons of qira ono mont aboog eed indicator yh from West eril to Inioqbim srij enisinoo MA (6) 08 bas 8A aldao ni nolent or animaleb.gniweb slepe eld 260 km/h D 1 D. e 51arrow_forward
- The figure gives the acceleration a versus time t for a particle moving along an x axis. The a-axis scale is set by as = 12.0 m/s². At t = -2.0 s, the particle's velocity is 11.0 m/s. What is its velocity at t = 6.0 s? a (m/s²) as -2 0 2 t(s) 4arrow_forwardTwo solid cylindrical rods AB and BC are welded together at B and loaded as shown. Knowing that the average normal stress must not exceed 150 MPa in either rod, determine the smallest allowable values of the diameters d₁ and d2. Take P= 85 kN. P 125 kN B 125 kN C 0.9 m 1.2 m The smallest allowable value of the diameter d₁ is The smallest allowable value of the diameter d₂ is mm. mm.arrow_forwardWestros, from Game of Thrones, has an area of approximately 6.73⋅106 miles26.73⋅106miles2. Convert the area of Westros to km2 where 1.00 mile = 1.609 km.arrow_forward
- a) What is the lenght of x? b) Findθ c) Find ϕarrow_forwardA surveyor measures the distance across a straight river by the following method: Starting directly across from a tree on the opposite bank, he walks x = 97.7 m along the riverbank to establish a baseline. Then he sights across to the tree. The angle from his baseline to the tree is θ = 33.0 °. How wide is the river?arrow_forwardA small turtle moves at a speed of 697. furlong/fortnight. Find the speed of the turtle in centimeters per second. Note that 1.00 furlong = 220. yards, 1.00 yard = 3.00 feet, 1.00 foot = 12.0 inches, 1.00 inch = 2.54 cm, and 1.00 fortnight = 14.0 days.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY