
(a)
The PV diagram for the cycle.
(a)
Answer to Problem 84P
The required PV diagram is shown in Figure 1.
Explanation of Solution
Formula:
The range of the isobaric expansion cycle is given by,
The range of the adiabatic compression cycle is given by,
The range for the isobaric compression cycle is given by,
The range of the adiabatic expansion cycle is given by,
Calculation:
The state functions A for the PV graph are given by,
The state functions B for the PV graph are given by,
The state functions C for the PV graph are given by,
The state functions D for the PV graph are given by,
The required PV diagram is shown in Figure 1
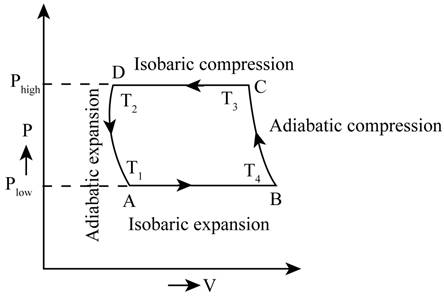
Figure 1
Conclusion:
Therefore, the required PV diagram is shown in Figure 1
(b)
The expression for the coefficient of performance.
(b)
Answer to Problem 84P
The value of
Explanation of Solution
Formula Used:
The expression for the coefficient of performance is given by,
The expression for
The expression for the value of
The expression for the work done during the cycle is given by,
The expression for the heat released in the entire cycle is given by,
The expression to determine the value of
The expression to determine the value of
Calculation:
The expression for the value of the
The difference between the value of
The value of
Conclusion:
Therefore, the value of
(c)
The coefficient of performance of the refrigerator.
(c)
Answer to Problem 84P
The value of value of
Explanation of Solution
Given:
The volume
The initial pressure
The volume
The temperature
The pressure ratio
Formula:
The expression for the temperature and the pressure after adiabatic process BC is given by,
The expression for the temperature and the pressure after adiabatic process DA is given by,
From above and from equation (1) the value of
The expression for
Calculation:
The value of
Conclusion:
Therefore, the value of
(d)
The electrical energy that must be supplied to the motor of rotor is given by,
(d)
Answer to Problem 84P
The electrical energy that must be supplied is
Explanation of Solution
Given:
The rate
Formula:
The expression to determine the electrical energy that must be supplied is given by,
Calculation:
The electrical energy that must be supplied is calculated as,
Conclusion:
Therefore, the electrical energy that must be supplied is
(e)
The amount added to the monthly electricity bill.
(e)
Answer to Problem 84P
The electrical energy that must be supplied is
Explanation of Solution
Given:
The number of days
The time for which the motor runs is
The charge
Formula:
The amount of electrical energy utilized by the refrigerator per month is given by,
Calculation:
The electrical energy utilized by the refrigerator per month is calculated as,
Conclusion:
Therefore, the electrical energy that must be supplied is
Want to see more full solutions like this?
Chapter 19 Solutions
Physics for Scientists and Engineers
- Three slits, each separated from its neighbor by d = 0.06 mm, are illuminated by a coherent light source of wavelength 550 nm. The slits are extremely narrow. A screen is located L = 2.5 m from the slits. The intensity on the centerline is 0.05 W. Consider a location on the screen x = 1.72 cm from the centerline. a) Draw the phasors, according to the phasor model for the addition of harmonic waves, appropriate for this location. b) From the phasor diagram, calculate the intensity of light at this location.arrow_forwardA Jamin interferometer is a device for measuring or for comparing the indices of refraction of gases. A beam of monochromatic light is split into two parts, each of which is directed along the axis of a separate cylindrical tube before being recombined into a single beam that is viewed through a telescope. Suppose we are given the following, • Length of each tube is L = 0.4 m. • λ= 598 nm. Both tubes are initially evacuated, and constructive interference is observed in the center of the field of view. As air is slowly let into one of the tubes, the central field of view changes dark and back to bright a total of 198 times. (a) What is the index of refraction for air? (b) If the fringes can be counted to ±0.25 fringe, where one fringe is equivalent to one complete cycle of intensity variation at the center of the field of view, to what accuracy can the index of refraction of air be determined by this experiment?arrow_forward1. An arrangement of three charges is shown below where q₁ = 1.6 × 10-19 C, q2 = -1.6×10-19 C, and q3 3.2 x 10-19 C. 2 cm Y 93 92 91 X 3 cm (a) Calculate the magnitude and direction of the net force on q₁. (b) Sketch the direction of the forces on qiarrow_forward
- (Figure 1)In each case let w be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w Find the direction of the force exerted on the strut by the pivot in the arrangement (a). Express your answer in degrees. Find the tension Tb in the cable in the arrangement (b). Express your answer in terms of w. Find the magnitude of the force exerted on the strut by the pivot in the arrangement (b). Express your answer in terms of w.arrow_forward(Figure 1)In each case let ww be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w. Find the direction of the force exerted on the strut by the pivot in the arrangement (b). Express your answer in degrees.arrow_forwardA 70.0 cm, uniform, 40.0 N shelf is supported horizontally by two vertical wires attached to the sloping ceiling (Figure 1). A very small 20.0 N tool is placed on the shelf midway between the points where the wires are attached to it. Find the tension in the left-hand wire. Express your answer with the appropriate units.arrow_forward
- Find the total bind Mev. binding energy for 13 Carbon, 6C (atomic mass = 13.0033554)arrow_forwardWhat is the 27 energy absorbed in this endothermic Auclear reaction 2] Al + 'n → 27 Mg + ! H? (The atom mass of "Al is 26.981539u. and that of 11 Mg is 26.984341u) MeVarrow_forwardWhat is the energy released in this nuclear reaction 1 F + "', H-1 O+ He? 19 19 16 (The atomic mass of 1F is 18.998403 u, and that of 20 is 15.9949154) MeV.arrow_forward
- What is the energy released in this B+ nuclear reaction خالد 2½ Al w/ Mg + ie? (The atomic mass of 11 Al is 23.9999394 and that > of 12 Mg is 23.985041 u) MeV.arrow_forwardWhat is the energy released / absorbed in this nuclear reaction 14 N+ & He → » O + ! N? (The atomic mass of 14 N is 14.003074u. 17N+ and that of 10 is 16.9991324). MeVarrow_forwardCan someone help me answer this question thanks.arrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





