
INTRO TO PHYSICAL SCIENCE W/MINDTAP
14th Edition
ISBN: 9781337077026
Author: Shipman
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 5AYK
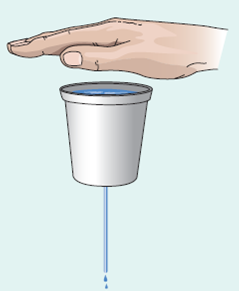
Water leaks from a cup with a hole, as shown in • Fig. 19.25. What would happen if you placed the palm of your hand tightly over the mouth of the cup? Why? (Hint: What happens to the pressure of the air above the water with respect to the pressure outside the cup?)
Figure 19.25 Stop That Leak See Applying Your Knowledge Question 5.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
An electric power station that operates at 30 KV and uses
a 15:1 set step-up ideal transformer is producing 400MW
(Mega-Watt) of power that is to be sent to a big city
with only 2.0% loss. What
which is located 270 km
away
is the resistance of the Two wires that are
being used?
52
Slink, from Toy Story, is a slinky dog whose middle section is a giant spring with a spring constant of 10.9 N/m. Woody, who has a mass of 0.412 kg, grabs onto the tail end of Slink and steps off the bed (as shown in figure A) with no initial velocity and reaches the floor right as his velocity hits zero again (as shown in figure C).
The character Min Min from Arms was a DLC character added to Super Smash Bros. Min Min’s arms are large springs, with a spring constant of 8.53 ⋅ 10^3 N/m, which she uses to punch and fling away her opponents. Min Min pushes her spring arm against Steve, who is not moving, compressing it 1.20 m as shown in figure A. Steve has a mass of 81.6 kg. Assuming she uses only the spring to launch Steve, how fast is Steve moving when the spring is no longer compressed? As Steve goes flying away he goes over the edge of the level, as shown in figure C. What is the magnitude of Steve’s velocity when he is 2.00 m below where he started?
Chapter 19 Solutions
INTRO TO PHYSICAL SCIENCE W/MINDTAP
Ch. 19.1 - What are the four major gaseous constituents of...Ch. 19.1 - Prob. 2PQCh. 19.2 - Prob. 1PQCh. 19.2 - Prob. 2PQCh. 19.3 - Prob. 1PQCh. 19.3 - Prob. 2PQCh. 19.3 - Prob. 19.1CECh. 19.4 - Prob. 1PQCh. 19.4 - Prob. 2PQCh. 19.5 - Prob. 1PQ
Ch. 19.5 - Prob. 2PQCh. 19 - KEY TERMS 1. atmospheric science (Intro) 2....Ch. 19 - Prob. BMCh. 19 - Prob. CMCh. 19 - Prob. DMCh. 19 - Prob. EMCh. 19 - KEY TERMS 1. atmospheric science (Intro) 2....Ch. 19 - Prob. GMCh. 19 - Prob. HMCh. 19 - Prob. IMCh. 19 - Prob. JMCh. 19 - Prob. KMCh. 19 - Prob. LMCh. 19 - Prob. MMCh. 19 - Prob. NMCh. 19 - Prob. OMCh. 19 - Prob. PMCh. 19 - Prob. QMCh. 19 - KEY TERMS 1. atmospheric science (Intro) 2....Ch. 19 - Prob. SMCh. 19 - Prob. TMCh. 19 - Prob. UMCh. 19 - Prob. VMCh. 19 - Prob. WMCh. 19 - Prob. XMCh. 19 - Prob. YMCh. 19 - Prob. ZMCh. 19 - Prob. AAMCh. 19 - Prob. BBMCh. 19 - Prob. CCMCh. 19 - Prob. DDMCh. 19 - Prob. 1MCCh. 19 - Prob. 2MCCh. 19 - Photosynthesis is responsible for the atmospheric...Ch. 19 - What regulates the Earths average temperature?...Ch. 19 - Prob. 5MCCh. 19 - Prob. 6MCCh. 19 - Prob. 7MCCh. 19 - Prob. 8MCCh. 19 - Prob. 9MCCh. 19 - Prob. 10MCCh. 19 - What is the cloud root name that means heap?...Ch. 19 - Prob. 12MCCh. 19 - Prob. 1FIBCh. 19 - Prob. 2FIBCh. 19 - Prob. 3FIBCh. 19 - Prob. 4FIBCh. 19 - One standard atmosphere of pressure is ___ lb2....Ch. 19 - Prob. 6FIBCh. 19 - Prob. 7FIBCh. 19 - Prob. 8FIBCh. 19 - Prob. 9FIBCh. 19 - Prob. 10FIBCh. 19 - Prob. 11FIBCh. 19 - Prob. 12FIBCh. 19 - Prob. 1SACh. 19 - Prob. 2SACh. 19 - Prob. 3SACh. 19 - Prob. 4SACh. 19 - Prob. 5SACh. 19 - Prob. 6SACh. 19 - Prob. 7SACh. 19 - Prob. 8SACh. 19 - Prob. 9SACh. 19 - In terms of Rayleigh scattering, why is it...Ch. 19 - Prob. 11SACh. 19 - Prob. 12SACh. 19 - What are the four fundamental atmospheric...Ch. 19 - Prob. 14SACh. 19 - Prob. 15SACh. 19 - When is the relative humidity 100%? It may be...Ch. 19 - Which way, relative to the wind direction, does a...Ch. 19 - Prob. 18SACh. 19 - Prob. 19SACh. 19 - What is a convection cycle, and what are the...Ch. 19 - Prob. 21SACh. 19 - Why does weather generally move from west to east...Ch. 19 - Prob. 23SACh. 19 - Prob. 24SACh. 19 - Name the cloud family for each of the following:...Ch. 19 - Prob. 26SACh. 19 - Prob. 27SACh. 19 - Why are clouds generally white, while some are...Ch. 19 - Visualize the connections and give answers in the...Ch. 19 - (a) Why does the land lose heat more quickly at...Ch. 19 - Prob. 2AYKCh. 19 - Prob. 3AYKCh. 19 - Prob. 4AYKCh. 19 - Water leaks from a cup with a hole, as shown in ...Ch. 19 - Express the approximate thicknesses of the (a)...Ch. 19 - Prob. 2ECh. 19 - If the air temperature is 70F at sea level, then...Ch. 19 - If the air temperature is 20C at sea level, then...Ch. 19 - On a day when the air temperature is 85F, the...Ch. 19 - Prob. 6ECh. 19 - On a very hot day with an air temperature of 105F,...Ch. 19 - On a winter day, a psychrometer has a dry-bulb...Ch. 19 - The dry-bulb and wet-bulb thermometers of a...Ch. 19 - Prob. 10ECh. 19 - On a day when the air temperature is 70F, a fellow...Ch. 19 - On another day with the same air temperature...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Calculate the energy needed to melt 50 g of 0°C icearrow_forwardTwo very long line charges are set up along lines that areparallel to the z-axis, so they set up Electric fields strictly in the xy plane. One goes throughthe x-axis at x = −0.40 m and has charge a density λ1 = +12.0 μC/m, the other goesthrough the x-axis at x = +0.40 m has charge density λ2 = −8.0 μC/m.A. Find the Electric field at point A: (0.40, 0.80) (distances in meters). Give answersin unit vector notation and draw a graph of the x-y plane with the E-fields you justfound.B. Find a point on the x-axis at which the total E-field is 0.arrow_forwardIn order to increase the amount of exercise in her daily routine, Tara decides to walk up the four flights of stairs to her car instead of taking the elevator. Each of the steps she takes are 18.0 cm high, and there are 12 steps per flight. (a) If Tara has a mass of 77.0 kg, what is the change in the gravitational potential energy of the Tara-Earth system (in J) when she reaches her car? ] (b) If the human body burns 1.5 Calories (6.28 x 10³ J) for each ten steps climbed, how much energy (in J) has Tara burned during her climb? ] (c) How does the energy she burned compare to the change in the gravitational potential energy of the system? Eburned Δυarrow_forward
- A 4.40 kg steel ball is dropped onto a copper plate from a height of 10.0 m. If the ball leaves a dent 2.75 mm deep, what is the average force exerted by the plate on the ball during the impact? Narrow_forwardA block of mass m = 7.00 kg is released from rest from point and slides on the frictionless track shown in the figure below. (Assume h₂ = 7.80 m.) a m ha 3.20 m 2.00 m i (a) Determine the block's speed at points ® and point B ©. m/s m/s point (b) Determine the net work done by the gravitational force on the block as it moves from point J A to pointarrow_forwardA 1.10 x 10²-g particle is released from rest at point A on the inside of a smooth hemispherical bowl of radius R R B 2R/3 (a) Calculate its gravitational potential energy at A relative to B. ] (b) Calculate its kinetic energy at B. ] (c) Calculate its speed at B. m/s (d) Calculate its potential energy at C relative to B. J (e) Calculate its kinetic energy at C. ] = 26.5 cm (figure below).arrow_forward
- Report on the percentage errors (with uncertainty) between the value of 'k' from the F vs displacement plot and each of the values of 'k' from the period measurements. Please comment on the goodness of the results. Value of k = Spring constant k = 50.00 N/m Each of the values of k from period measurements: Six Measurements of time for 5 osccilations: t1 = 7.76s, t2=8.00s, t3=7.40s, t4=7.00s, t5=6.90s, t6=7.10s (t1-tavg)^2 = (7.76-7.36)^2 = 0.16%(t2-tavg)^2 =(8.00-7.36)^2 = 0.4096%(t3-tavg)^2 =(7.40-7.36)^2 = 0.0016%(t4-tavg)^2 =(7.00-7.36)^2 = 0.1296%(t5-tavg)^2 =(6.90-7.36)^2 = 0.2116%(t6-tavg)^2 =(7.10-7.36)^2 = 0.0676arrow_forwardNo chatgpt pls will upvotearrow_forwardBased on the two periods (from hand timed and ultrasonic sensor), find the value of 'k' they suggest from the physics and from the value of the hanging mass. hand time period is 1.472s and ultrasonic sensor time period is 1.44sarrow_forward
- No chatgpt pls will upvotearrow_forwardExperimental Research Report Template Title: Paper Airplane Flight. Materials: Paper, ruler, tape Procedure: Fold paper into different airplane designs, such as dart, glider, or classic. Measure and record the distances each design flies when thrown with the same force. Discuss aerodynamics and the factors that affect flight distance. Introduction: (What do you expect to learn? What is the purpose of this lab? List any questions this experiment will answer.) Hypothesis: (Predict the outcome(s) of the experiment, must be in an “if…then format.) Materials: (What equipment and materials did you need for this experiment assignment? Describe how any equipment was connected. Also mention any special hardware or connections. List the name and amount of each item used.) Procedures: (What steps did you take to accomplish this lab assignment? Include Safety Precautions.) Data Collection: (Record the data that is required at each step of the…arrow_forwardTitle: Studying the Relationship Between Drop Height and Bouncing Height of a Ball: You can drop balls of different materials (e.g., rubber, plastic, ping pong) from various heights onto a flat surface and measure the height of their bounce using a ruler. Introduction: (What do you expect to learn? What is the purpose of this lab? List any questions this experiment will answer.) Hypothesis: (Predict the outcome(s) of the experiment, must be in an “if…then format.) Materials: (What equipment and materials did you need for this experiment assignment? Describe how any equipment was connected. Also mention any special hardware or connections. List the name and amount of each item used.) Procedures: (What steps did you take to accomplish this lab assignment? Include Safety Precautions.) Data Collection: (Record the data that is required at each step of the lab: tables, charts, graphs, sketches, etc.) Data Analysis: (Explain you…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Newton's First Law of Motion: Mass and Inertia; Author: Professor Dave explains;https://www.youtube.com/watch?v=1XSyyjcEHo0;License: Standard YouTube License, CC-BY