
Interpretation:
For each of the following reaction the route that could reasonably be expected from the starting material to the product is to be provided.
Concept introduction:
Electrophiles are electron deficient species that have positive or partially positive charge. Lewis acids are electrophiles that accept electron pair.
Nucleophiles are electron rich species that have negative or partially negative charge. Lewis bases are nucleophiles that donate electron pair.
Free radical is an atom, molecule, or ion that has an unpaired electron, which makes it highly chemically reactive.
Substitution reaction: A reaction in which one of the hydrogen atoms of a hydrocarbon or a
Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed is called elimination reaction.
Addition reaction: It is the reaction in which unsaturated bonds are converted to saturated molecules by the addition of molecules.
The condensation reactions in carbonyl, the enolate or enol of a carbonyl group of one compound reacts with the carbonyl group of another compound. The two main types of condensation are Claisen and aldol condensation.
Claisen condensation entails the formation of 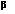 keto esters through carbon–carbon bond formation.
keto esters through carbon–carbon bond formation.
The Claisen condensation is the carbon–carbon bond formation reaction and is important for the preparation of
In Claisen condensation, the ester of one molecule is added to the carbonyl carbon of another molecule and the formation of
When one ester molecule has no alpha hydrogen, crossed Claisen condensation occurs.
Aldol condensation involves the reaction of enolate of an 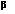 hydroxyl ketone or aldehyde.
hydroxyl ketone or aldehyde.
An aldol reaction takes place by acid catalysis, and direct dehydration of
A
A chemical reaction that is catalyzed by an base is called base catalyzed reaction.
Aldol reaction is preferred in basic condition over acidic condition as after the aldol condensation, acid catalysis promotes the reaction further.
A carbon–carbon bond formation occurs through aldol condensation reaction.
An aldol reaction takes place in a protic solvent with a base.
Dehydration of aldol addition product leads to the formation of conjugated
Trending nowThis is a popular solution!

Chapter 19 Solutions
EBK ORGANIC CHEMISTRY
- What is the major product of the following reaction? O IV III HCI D = III ა IVarrow_forwardThe reaction of what nucleophile and substrate is represented by the following transition state? CH3 CH3O -Br อ δ CH3 Methanol with 2-bromopropane Methanol with 1-bromopropane Methoxide with 1-bromopropane Methoxide with 2-bromopropanearrow_forwardWhat is the stepwise mechanism for this reaction?arrow_forward
- 32. Consider a two-state system in which the low energy level is 300 J mol 1 and the higher energy level is 800 J mol 1, and the temperature is 300 K. Find the population of each level. Hint: Pay attention to your units. A. What is the partition function for this system? B. What are the populations of each level? Now instead, consider a system with energy levels of 0 J mol C. Now what is the partition function? D. And what are the populations of the two levels? E. Finally, repeat the second calculation at 500 K. and 500 J mol 1 at 300 K. F. What do you notice about the populations as you increase the temperature? At what temperature would you expect the states to have equal populations?arrow_forward30. We will derive the forms of the molecular partition functions for atoms and molecules shortly in class, but the partition function that describes the translational and rotational motion of a homonuclear diatomic molecule is given by Itrans (V,T) = = 2πmkBT h² V grot (T) 4π²IKBT h² Where h is Planck's constant and I is molecular moment of inertia. The overall partition function is qmolec Qtrans qrot. Find the energy, enthalpy, entropy, and Helmholtz free energy for the translational and rotational modes of 1 mole of oxygen molecules and 1 mole of iodine molecules at 50 K and at 300 K and with a volume of 1 m³. Here is some useful data: Moment of inertia: I2 I 7.46 x 10- 45 kg m² 2 O2 I 1.91 x 101 -46 kg m²arrow_forwardK for each reaction step. Be sure to account for all bond-breaking and bond-making steps. HI HaC Drawing Arrows! H3C OCH3 H 4 59°F Mostly sunny H CH3 HO O CH3 'C' CH3 Select to Add Arrows CH3 1 L H&C. OCH3 H H H H Select to Add Arrows Q Search Problem 30 of 20 H. H3C + :0: H CH3 CH3 20 H2C Undo Reset Done DELLarrow_forward
- Draw the principal organic product of the following reaction.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided structures, draw the curved arrows that epict the mechanistic steps for the proton transfer between a hydronium ion and a pi bond. Draw any missing organic structures in the empty boxes. Be sure to account for all lone-pairs and charges as well as bond-breaking and bond-making steps. 2 56°F Mostly cloudy F1 Drawing Arrows > Q Search F2 F3 F4 ▷11 H. H : CI: H + Undo Reset Done DELLarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbons. Draw out the benzene ring structure when doing itarrow_forward
- 1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series. 2) Calculate the ionization energy of He* and L2+ ions in their ground states. 3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.arrow_forwardCalculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forwardPlleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forward
