
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
8th Edition
ISBN: 9780134581064
Author: Bruice, Paula Yurkanis
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 43P
Organic chemists work with tetraphenylporphyrins rather than with porphyrins because tetraphenylporphyrins are much more resistant to air oxidation, Tetraphenylporphyrin can be prepared by the reaction of benzaldehyde with pyrrole. Please a mechanis, for the formation of the ring system shown here:
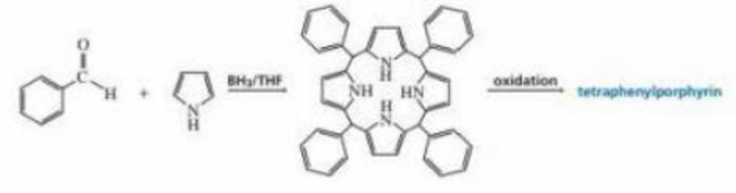
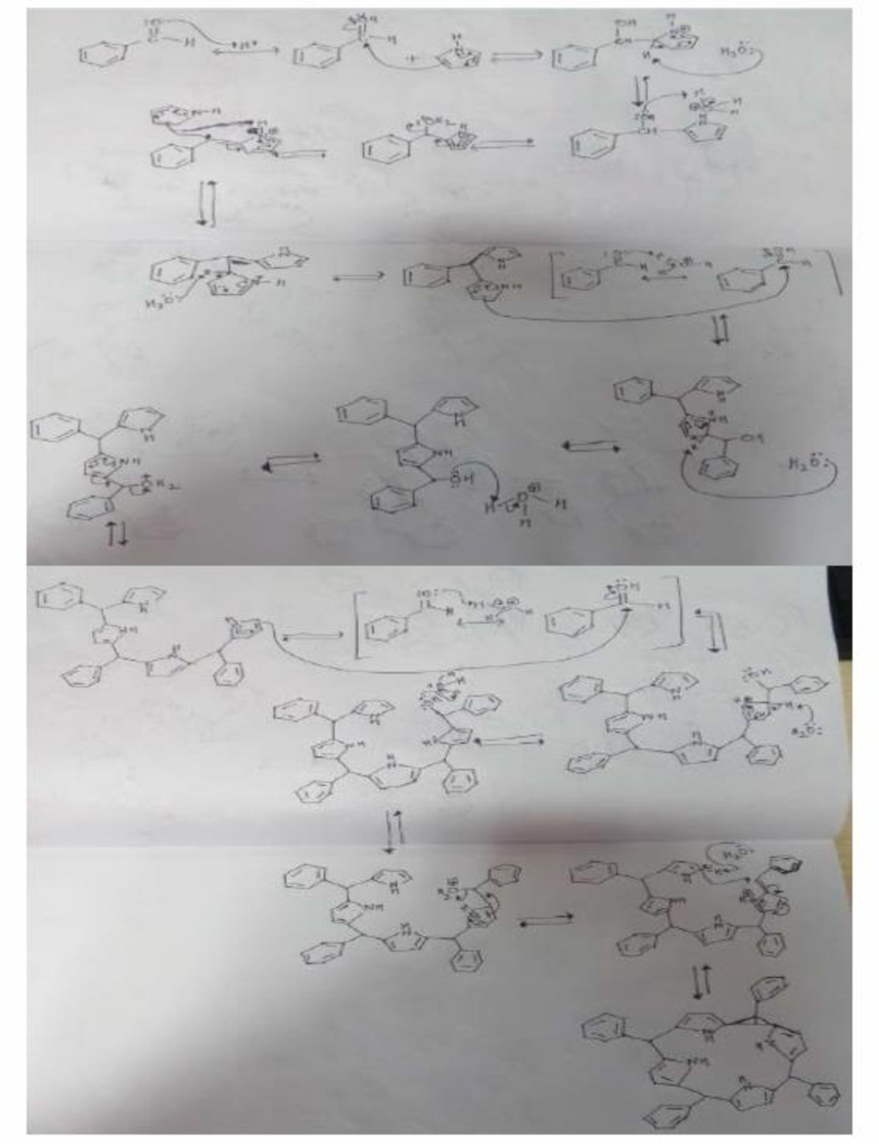
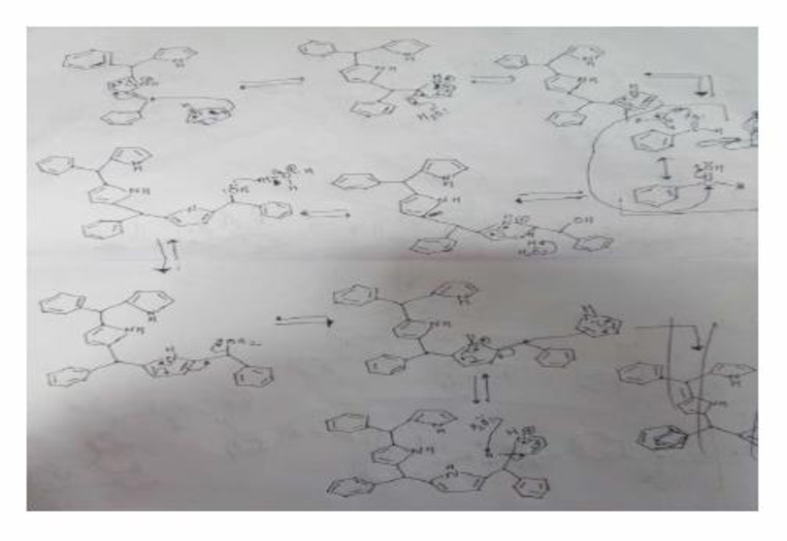
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 19 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
Ch. 19.1 - Name the following:Ch. 19.2 - Prob. 3PCh. 19.2 - Prob. 4PCh. 19.3 - Draw the product of each of the following...Ch. 19.5 - Prob. 6PCh. 19.5 - Explain why cyclopentadiene (pKa = 15) is more...Ch. 19.5 - When pyrrole is added to a dilute solution of...Ch. 19.6 - Prob. 10PCh. 19.6 - How to the mechanisms of the following reactions...Ch. 19.6 - Prob. 12P
Ch. 19.6 - Rank the following compounds from easiest to...Ch. 19.7 - Prob. 14PCh. 19.7 - Prob. 15PCh. 19.7 - Prob. 16PCh. 19.7 - Prob. 17PCh. 19.7 - Prob. 18PCh. 19.7 - Prob. 19PCh. 19.7 - Prob. 20PCh. 19 - Name the following:Ch. 19 - Prob. 22PCh. 19 - Rank the following compounds from strongest acid...Ch. 19 - Which of the following compounds is easier to...Ch. 19 - Rank the following compounds from most reactive to...Ch. 19 - One of the following compounds undergoes...Ch. 19 - Benzene undergoes electrophilic aromatic...Ch. 19 - Pyrrole reacts with excess...Ch. 19 - The dipole moments of furan and tetrahydrofuran...Ch. 19 - Name the following:Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - a. Draw resonance contributors to show why...Ch. 19 - The chemical shifts of the C-2 hydrogen in the...Ch. 19 - Explain why protonating aniline has a dramatic...Ch. 19 - Prob. 36PCh. 19 - Propose a mechanism for the following reaction:Ch. 19 - Prob. 38PCh. 19 - Propose a mechanism for the following reactions:Ch. 19 - Prob. 40PCh. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - Organic chemists work with tetraphenylporphyrins...Ch. 19 - Show how the following compounds can be prepared...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License