
Chemistry for Changing Times
14th Edition
ISBN: 9780134212777
Author: John W. Hill; Terry W. McCreary
Publisher: Pearson Education (US)
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 19, Problem 28P
Interpretation Introduction
Interpretation: The amount of protein required everyday by a 110 lb gymnast when DRI for protein is 0.8 g/kg should be determined.
Concept introduction:
- DRI is the dietary reference intake. It is different for fat, protein and carbohydrates.
- If weight of the man is X kg, of energy and DRI of a nutrient is Y g/kg, then the amount of nutrient required by the man is
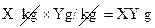
- 1 lb =0.45kg
Given:
Weight of the gymnast = 110 lb
DRI for protein = 0.8 g/kg
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.
a. The first three lines of this procedure describe the reaction used to make compound 5b. In the fourth line, hexane and sodium bicarbonate are added. What organic lab technique is being used here?
b. What is the purpose of the Na2SO4?
c. What equipment would you use to “concentrate [a solution] under reduced pressure”?
When N,N-dimethylaniline is treated with bromine both the ortho and para products
are observed. However when treated with a mixture of nitric acid and sulfuric acid only
the meta product is observed. Explain these results and support your answer with the
appropriate drawings *Hint amines are bases*
N
HNO3
H2SO4
N
NO2
N
Br2
N
Br
+
N
8-8-8
FeBr3
Br
Chapter 19 Solutions
Chemistry for Changing Times
Ch. 19 - Prob. 1RQCh. 19 - Prob. 2RQCh. 19 - Prob. 3RQCh. 19 - 4. Do you think it is likely that obesity is...Ch. 19 - Prob. 5RQCh. 19 - Prob. 6RQCh. 19 - Prob. 7RQCh. 19 - Prob. 8RQCh. 19 - Prob. 9RQCh. 19 - Prob. 10RQ
Ch. 19 - What are the common dietary needs for athletes?...Ch. 19 - Prob. 12PCh. 19 - Prob. 13PCh. 19 - Prob. 14PCh. 19 - Prob. 15PCh. 19 - Prob. 16PCh. 19 - Prob. 17PCh. 19 - Prob. 18PCh. 19 - Prob. 19PCh. 19 - Prob. 20PCh. 19 - Prob. 21PCh. 19 - Prob. 22PCh. 19 - Prob. 23PCh. 19 - Prob. 24PCh. 19 - Prob. 25PCh. 19 - Prob. 26PCh. 19 - Prob. 27PCh. 19 - Prob. 28PCh. 19 - Prob. 29PCh. 19 - Prob. 30PCh. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - Prob. 33PCh. 19 - Prob. 34PCh. 19 - Prob. 35PCh. 19 - Prob. 36PCh. 19 - Prob. 37PCh. 19 - Prob. 38PCh. 19 - Prob. 39PCh. 19 - Prob. 40PCh. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - Prob. 43PCh. 19 - Prob. 44PCh. 19 - Prob. 45PCh. 19 - Prob. 46PCh. 19 - Prob. 47PCh. 19 - Prob. 48PCh. 19 - Prob. 49PCh. 19 - Prob. 50PCh. 19 - Prob. 51PCh. 19 - Prob. 52PCh. 19 - Prob. 53PCh. 19 - 54. Why is it important for pregnant women not to...Ch. 19 - Prob. 55APCh. 19 - Prob. 56APCh. 19 - Prob. 57APCh. 19 - Prob. 58APCh. 19 - Prob. 59APCh. 19 - Prob. 60APCh. 19 - Prob. 61APCh. 19 - Prob. 62APCh. 19 - Prob. 63APCh. 19 - Prob. 64APCh. 19 - Prob. 65APCh. 19 - Prob. 66APCh. 19 - Prob. 67APCh. 19 - Prob. 19.1CTECh. 19 - Prob. 19.2CTECh. 19 - 19.3 Consuming large doses of vitamins and...Ch. 19 - Prob. 19.4CTECh. 19 - An online advertisement says. I cut down 47 lb of...Ch. 19 - Prob. 19.6CTECh. 19 - Prob. 1CGPCh. 19 - Prob. 2CGPCh. 19 - Prob. 3CGPCh. 19 - Prob. 4CGPCh. 19 - Prob. 5CGPCh. 19 - Prob. 1CHQCh. 19 - Prob. 2CHQCh. 19 - Prob. 3CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a mechanism that explains the formation of compound OMe SO3H 1. Fuming H2SO4arrow_forwardConsider the following two acid-base reactions: OH OHI Based on what you know about the compounds and their acidity, which direction would you expect both of these reactions to proceed? Show your reasoning. A pKa table has been provided in case you need it. Functional group Example pka CHA -50 Alkane -35 Amine : NH3 Alkyne RH 25 Water HO-H 169 16 10 Protonated amines NH 10 5 Carboxylic acids OH Hydrochloric acid HCI A chemist intends to run the following reaction on the three substrates shown below: H₂O R-CI product room temp. Cl Cl (1) (2) (3) They find one will react quickly, one slowly, and one will not react at all. Which is which, and why? HINT: What is the reaction they're trying to do? Does that mechanism tell you anything about why something would be favored?arrow_forwardNH3 decomposes through an equilibrium reaction between NH3, H2, and N2. Only one of the options is correct:(A). The mechanism of the NH3 decomposition reaction must necessarily involve the collision of two NH3 molecules to induce a rearrangement of the atoms in this molecule.(B). The molecular weight of the NH3 decomposition reaction is 2 since two NH3 molecules must collide.(C). The rate of the NH3 decomposition reaction must be greater than that of NH3 synthesis, since the former requires two molecules to collide and the latter, four.(D). The NH3 decomposition reaction cannot occur in a single step.arrow_forward
- Given the equilibrium A2 + B2 ⇌ 2 AB where k1 is the rate coefficient of the forward reaction and k-1 is the rate coefficient of the reverse reaction, with the forward reaction being first-order in A2 and B2, and the reverse reaction being second-order in AB. Equilibrium will be reached later if the relative values of the constants are:(A) k1 high and k-1 high(B) k1 high and k-1 low(C) k1 low and k-1 high(D) k1 low and k-1 lowarrow_forwardA 2-step reaction has the following mechanism: | 1. (fast) R2 R+R 2. (slow) R+Q K₂ P k_1 What series does it have? (A). v= - = (k + k1 − k-1)[R2][Q] (B). v=-k₁[R₂] + k₁[R]² - k₂[R][Q] (C). v=k₂[R]²[Q]² (D). v = k[R₂]1/2[Q]arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardLabel the α and ẞ carbons in each alkyl halide. Draw all possible elimination products formed when each alkyl halide is treated with K-OC(CH3), b. ان Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY