
In Figure P19.22, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is −500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D?
Figure P19.22
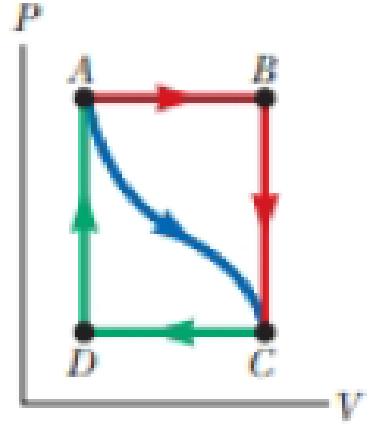
(c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D?
(a)
The energy which must be added to the system by heat which goes from A through B to C.
Answer to Problem 22P
The energy which must be added to the system by heat which goes from A through B to C is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Write the equation of conservation of energy.
Here,
Substitute
Substitute
Conclusion:
Therefore, the energy which must be added to the system by heat which goes from A through B to C is
(b)
The work done on the system from C to D if pressure at A is five times that of point C.
Answer to Problem 22P
The work done on the system from C to D if pressure at A is five times that of point C is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation to calculate the work done on the system from C to D.
Here,
Here,
The pressure at point A is five times that of point C.
And the volume is,
Substitute
Substitute
Conclusion:
Therefore, the work done on the system from C to D if pressure at A is five times that of point C is
(c)
The energy exchanged with the surroundings by heat along the green path from C to A.
Answer to Problem 22P
The energy exchanged with the surroundings by heat along the green path from C to A is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Write the equation to calculate work done along the green path from C to A.
Here, the volume
Substitute 0 for
Substitute
Substitute
Conclusion:
Therefore, the energy exchanged with the surroundings by heat along the green path from C to A is
(d)
The energy which must be added to the system by heat which goes from C to D.
Answer to Problem 22P
The energy which must be added to the system by heat which goes from C to D
is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Substitute
Conclusion:
Therefore, the energy which must be added to the system by heat which goes from C to D is
Want to see more full solutions like this?
Chapter 19 Solutions
Bundle: Physics For Scientists And Engineers With Modern Physics, 10th + Webassign Printed Access Card For Serway/jewett's Physics For Scientists And Engineers, 10th, Multi-term
- Which of the following best describes how to calculate the average acceleration of any object? Average acceleration is always halfway between the initial acceleration of an object and its final acceleration. Average acceleration is always equal to the change in velocity of an object divided by the time interval. Average acceleration is always equal to the displacement of an object divided by the time interval. Average acceleration is always equal to the change in speed of an object divided by the time interval.arrow_forwardThe figure shows the velocity versus time graph for a car driving on a straight road. Which of the following best describes the acceleration of the car? v (m/s) t(s) The acceleration of the car is negative and decreasing. The acceleration of the car is constant. The acceleration of the car is positive and increasing. The acceleration of the car is positive and decreasing. The acceleration of the car is negative and increasing.arrow_forwardWhich figure could represent the velocity versus time graph of a motorcycle whose speed is increasing? v (m/s) v (m/s) t(s) t(s)arrow_forward
- Unlike speed, velocity is a the statement? Poisition. Direction. Vector. Scalar. quantity. Which one of the following completesarrow_forwardNo chatgpt pls will upvote Already got wrong chatgpt answerarrow_forward3.63 • Leaping the River II. A physics professor did daredevil stunts in his spare time. His last stunt was an attempt to jump across a river on a motorcycle (Fig. P3.63). The takeoff ramp was inclined at 53.0°, the river was 40.0 m wide, and the far bank was 15.0 m lower than the top of the ramp. The river itself was 100 m below the ramp. Ignore air resistance. (a) What should his speed have been at the top of the ramp to have just made it to the edge of the far bank? (b) If his speed was only half the value found in part (a), where did he land? Figure P3.63 53.0° 100 m 40.0 m→ 15.0 marrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





