
Sketch and label a galvanic cell that makes use of the following spontaneous
Write the half-reactions for the anode and cathode. Give the standard cell notation. (Hint: Determine which half-reaction represents oxidation and which represents reduction.)
Interpretation:
For the given spontaneous redox reaction, a galvanic cell is to be labeled and sketched, the half- reactions for anode and cathode are to be written, and a standard cell notation is to be given.
Concept Introduction:
The electrode at which reduction occurs is known as cathode and the electrode at which oxidation occurs is known as anode.
The experimental apparatus that creates electric current from the spontaneous redox reaction is known as galvanic cell.
In the standard cell notation, the single vertical line represents the phase boundary and the double vertical line represents the salt bridge, the anode half-cell is described to the left of the double line and the cathode half-cell is described to the right of the double line.
Answer to Problem 1PE
Solution:
Cathode:
Anode:
Cell notation:
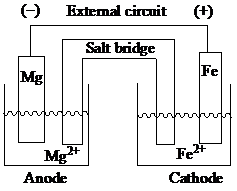
Explanation of Solution
The given spontaneous redox reaction is as:
At anode, oxidation takes place, whereas, at cathode, reduction takes place. In this galvanic cell, magnesium acts as the anode and iron acts as the cathode. In order to balance the redox reaction, iron gains two electrons while magnesium loses two electrons. Salt bridge enables the flow of ions which neutralize the charge imbalance. Within the salt bridge, the negative charge flows towards the anode to neutralize the accumulation of positive charge. Similarly, the positive charge flows towards the cathode to neutralize the accumulation of negative charge.
The half-cell reactions that take place at cathode and anode are as:
Half-cell reaction at cathode:
Half-cell reaction at anode:
The sketch of a galvanic cell is as:

In the standard cell notation, the magnesium anode half-cell is placed on the left and the cathode half-cell is placed on the right separated by the salt bridge. The standard cell notation for the given spontaneous redox reaction is represented as:
The half-cell reaction for anode and cathode and the standard notation cell notation have been written as:
Cathode:
Anode:
Cell notation:
Want to see more full solutions like this?
Chapter 19 Solutions
EBK CHEMISTRY: THE MOLECULAR NATURE OF
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Applications and Investigations in Earth Science (9th Edition)
Living By Chemistry: First Edition Textbook
Campbell Essential Biology (7th Edition)
- Draw the major products of the following reaction: HCIarrow_forwardFor each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral.arrow_forwardBlackboard app.aktiv.com X Organic Chemistry II Lecture (mx Aktiv Learning App Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 25 of 35 Select to Edit Arrows CH3CH2OK, CH3CH2OH L Gemini M 31 0:0 :0: 5x Undo Reset Done :0: Harrow_forward
- I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to me.I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to marrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Problem 17 of 35 1. CH3CH2Li O H 2. Neutralizing work-up @ Atoms, Bonds and Rings Draw or tap a new boarrow_forwardWill this convert the C=O to an alcohol? Or does its participation in the carboxy group prevent that from happening?arrow_forward
- I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to me.I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to marrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardCould you explain and label how this was determined for the functional groups? Please highlight the areas and show me as well.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





