
Concept explainers
(a)
Interpretation: For the given cases number of moles amount of heat released, energy released for each cases for both decay should be determined.
Concept Introduction:
- Energy required to break the nucleus into its corresponding proton and neutron is called nuclear binding energy
- This quantity represents the conversion of mass to energy occurs during an exothermic reaction.
- Nuclear binding energy can be calculated by Einstein’s mass energy equivalence relationship that is,ΔE= (Δm) c2
-
Where, (Δm) is called mass defect.
- The difference between mass of an atom and the sum of the masses of its proton, electron, and neutron is called Mass defect
(a)
Answer to Problem 19.76QP
The energy released in the above two decays is 5.59×10-15 J and 2.84×10-13J.
The total amount of energy released is:
(5.59×10-15 J)+2.84×10-13J=2.90×10-13J
Explanation of Solution
In case of Sr90 decay, the mass defect is
Δm=(massY90+mass e-)-massSr90
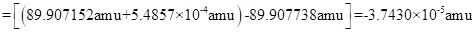
=(-)3.7430× 10-5 amu)×1g6.022×1023amu =6.216 10-29 g =-6.216×10-32kg
The energy change is given by :
ΔE(Δm)c2
(-6.216×10-32kg)(3.00×108m/s)2
=5.59×10-15kgm2/s2=5.59×10-15J
Similarly for Y90 decay, we have
Δm=(massZr90+masse-)-massY90
[(89.904703 amu + 5.4857 ×10-4amu-89.907152amu]=-1.9004×10-3amu
(-1.9004 ×10−3 amu)×1 g6.022×1023 amu=−3.156× 10−27g
=- 3.156× 10−30kg
And the energy change is given by:
ΔE=(-3.156×10-30kg)(3.00×108m/s)2=-2.84×10-13J
The energy released in the above two decays is 5.59×10-15 J and 2.84×10-13J.
The total amount of energy released is:
(5.59×10-15 J)+2.84×10-13J=2.90×10-13J
(b)
Interpretation: For the given cases number of moles amount of heat released, energy released for each cases for both decay should be determined.
Concept Introduction:
- Energy required to break the nucleus into its corresponding proton and neutron is called nuclear binding energy
- This quantity represents the conversion of mass to energy occurs during an exothermic reaction.
- Nuclear binding energy can be calculated by Einstein’s mass energy equivalence relationship that is,ΔE= (Δm) c2
-
Where, (Δm) is called mass defect.
- The difference between mass of an atom and the sum of the masses of its proton, electron, and neutron is called Mass defect
To calculate: The number of moles 90Sr decaying in a year.
(b)
Answer to Problem 19.76QP
The number of moles of nuclei which decay in a year is :
(1- 0.9756) mol = 0.0244 mol
This is a reasonable number since it takes 28.1 years for 0.5 moles of 90Sr to decay
Explanation of Solution
This calculation requires that we know the rate constant for the decay.
From half-life, we can calculate k.
k = 0.693t1/2=0.69328.1yr=0.0247 yr-1
To calculate the number of moles 90Sr decaying in a year, we apply the following equation:
ln NtN0=-kt
lnx1=-(0.0247yr-1)(1yr)
Where x is the number of moles nuclei left over .solving, we obtained the value of x
x = 0.9756 mol 90Sr
Thus the number of moles of nuclei which decay in a year is :
(1- 0.9756) mol = 0.0244 mol
This is a reasonable number since it takes 28.1 years for 0.5 moles of 90Sr to decay
(c)
Interpretation: For the given cases number of moles amount of heat released, energy released for each cases for both decay should be determined.
Concept Introduction:
- Energy required to break the nucleus into its corresponding proton and neutron is called nuclear binding energy
- This quantity represents the conversion of mass to energy occurs during an exothermic reaction.
- Nuclear binding energy can be calculated by Einstein’s mass energy equivalence relationship that is,ΔE= (Δm) c2
-
Where, (Δm) is called mass defect.
- The difference between mass of an atom and the sum of the masses of its proton, electron, and neutron is called Mass defect
To calculate: The heat released from 1 mole of 90Sr waste in a year.
(c)
Answer to Problem 19.76QP
The heat released from 1 mole of 90Sr waste in a year is given by:
heat released =(1.47× 1022nuclei)×2.90×10-13J1nucleus=4.26×109J=4.26×109kJ
Explanation of Solution
The half –life of Y90 is much shorter than that of 90Sr.
We can assume that Y90 formed from 90Sr and will be converted to Z90.
The energy changes in (a) refer to decay of individual nuclei.
In 0.0244 moles, the number of nuclei that have decay is:
0.0244 mol×6.022 ×10 23nuclei1mol=1.47×1022nuclei
There are two decay processes occurring, so we need to add the energy released for each process calculated in part (a).
So the heat released from 1 mole of 90Sr waste in a year is given by:
heat released =(1.47× 1022nuclei)×2.90×10-13J1nucleus=4.26×109J=4.26×109kJ
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry
- What would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forward
- Please complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forward
- What is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forwardWhat would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forward
- For benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





