
Concept explainers
(a)
Interpretation:
The percentage of atoms having velocity within
Concept introduction:
The probability distribution function of the velocities of the gas molecules in each dimension is given by
The Maxwell-Boltzmann distribution is shown below.
This distribution depends on the mass of the particle and absolute temperature.
Answer to Problem 19.73E
The percentage of atoms having a velocity within
Explanation of Solution
The given temperature is
The molar mass of the helium gas is
The graph of
Figure 1
The formula to calculate the root-mean-square speed is given below.
Where,
•
•
•
Substitute the values the value of the molar mass of helium,
The corresponding value of the percentage of atoms having
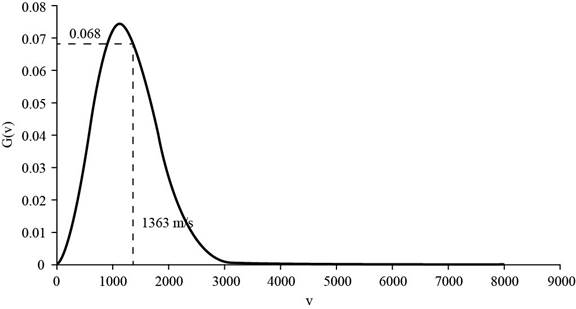
Figure 2
Therefore, the percentage of atoms having velocity within
The percentage of atoms having velocity within
(b)
Interpretation:
The percentage of atoms having velocity within
Concept introduction:
The probability distribution function of the velocities of the gas molecules in each dimension is given by
The Maxwell-Boltzmann distribution is given by,
This distribution depends on the mass of the particle and absolute temperature.
Answer to Problem 19.73E
The percentage of atoms having velocity within
Explanation of Solution
The given temperature is
The molar mass of the helium gas is
The graph of
Figure 1
The formula to calculate the most probable speed is given below as,
Where,
•
•
•
Substitute the values the value of the molar mass of helium,
The corresponding value of the percentage of atoms having
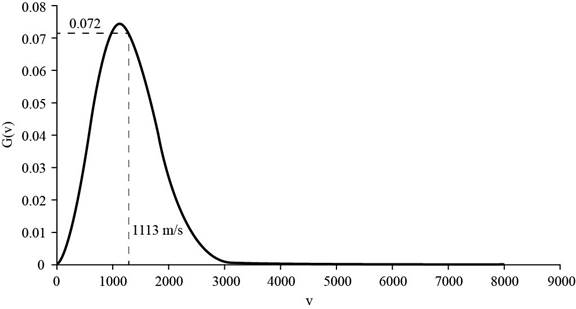
Figure 3
Therefore, the percentage of atoms having velocity within
The percentage of atoms having velocity within
(c)
Interpretation:
The percentage of atoms having velocity within
Concept introduction:
The probability distribution function of the velocities of the gas molecules in each dimension is given by
The Maxwell-Boltzmann distribution is given by,
This distribution depends on the mass of the particle and absolute temperature.
Answer to Problem 19.73E
The percentage of atoms having velocity within
Explanation of Solution
The given temperature is
The molar mass of the helium gas is
The graph of
Figure 1
The formula to calculate the mean speed is given below as,
Where,
•
•
•
Substitute the values the value of molar mass of helium,
The corresponding value of percentage of atoms having
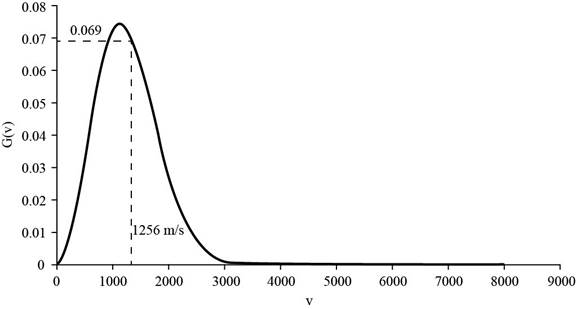
Figure 4
Therefore, the percentage of atoms having velocity within
The percentage of atoms having velocity within
The percentage of atoms having velocity within
Therefore, all the percentages have a relative same value.
The percentage of atoms having velocity within
Want to see more full solutions like this?
Chapter 19 Solutions
Physical Chemistry
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardhelp with the rf values i am so confusedarrow_forwardPredict the organic reactant of X and Y that are involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forwardPredict the major organic product for this reaction.arrow_forward
- help me with the rf value i am so confusedarrow_forwardPredict the major organic product for this reaction.arrow_forward3) The following molecule, chloral is a common precursor to chloral hydrate, an acetal type molecule that was a first-generation anesthetic. Draw a mechanism that accounts for tis formation and speculate why it does not require the use of an acid catalyst, like most hemiacetal and acetal reaction: (10 pts) H H₂Oarrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





