
PRINCIPLES OF INSTRUMENTAL ANALYSIS
7th Edition
ISBN: 9789353506193
Author: Skoog
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 19.36QAP
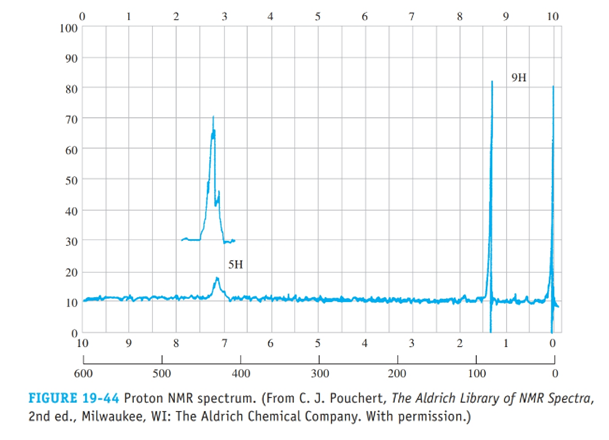
From the proton NMR spectrum in Figure 19-44, deduce the structure of this hydrocarbon
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Electrochemistry. Briefly describe the Donnan potential.
Indicate what the Luther equation is used for?
Indicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.
Chapter 19 Solutions
PRINCIPLES OF INSTRUMENTAL ANALYSIS
Ch. 19 - Prob. 19.1QAPCh. 19 - Prob. 19.2QAPCh. 19 - Prob. 19.3QAPCh. 19 - Prob. 19.4QAPCh. 19 - Prob. 19.5QAPCh. 19 - A nucleus has a spin quantum number of 7/2. How...Ch. 19 - Prob. 19.7QAPCh. 19 - Prob. 19.8QAPCh. 19 - Prob. 19.9QAPCh. 19 - Why is 133C-133C spin-spin splitting not observed...
Ch. 19 - Prob. 19.11QAPCh. 19 - Prob. 19.12QAPCh. 19 - Prob. 19.13QAPCh. 19 - What is a rotating frame of reference?Ch. 19 - How will E for an isolated 13C nucleus compare...Ch. 19 - Prob. 19.16QAPCh. 19 - Prob. 19.17QAPCh. 19 - Prob. 19.18QAPCh. 19 - Prob. 19.19QAPCh. 19 - Prob. 19.20QAPCh. 19 - Prob. 19.21QAPCh. 19 - Prob. 19.22QAPCh. 19 - Prob. 19.23QAPCh. 19 - Prob. 19.24QAPCh. 19 - Prob. 19.25QAPCh. 19 - Prob. 19.26QAPCh. 19 - Prob. 19.27QAPCh. 19 - Prob. 19.28QAPCh. 19 - Prob. 19.29QAPCh. 19 - Prob. 19.30QAPCh. 19 - The proton NMR spectrum in Figure 19.39 is for an...Ch. 19 - The proton NMR spectrum in Figure 19-40 is for a...Ch. 19 - Prob. 19.33QAPCh. 19 - Prob. 19.34QAPCh. 19 - Prob. 19.35QAPCh. 19 - From the proton NMR spectrum in Figure 19-44,...Ch. 19 - From the proton spectrum given in Figure 19-45,...Ch. 19 - Prob. 19.38QAPCh. 19 - Prob. 19.39QAPCh. 19 - Prob. 19.40QAPCh. 19 - Prob. 19.41QAPCh. 19 - Prob. 19.42QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forward
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY