
(a)
Interpretation:
The compound should be identified using the following proton NMR spectra whose empirical formula is C8H10:
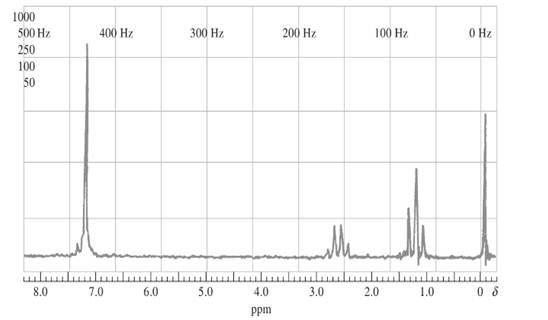
Concept introduction:
Proton NMR is application of nuclear magnetic resonance with respect to 1H nuclei in molecules in order to determine structure. Protons that are in the same magnetic environment are considered as chemically equivalent protons.
(b)
Interpretation:
The compound should be identified using the following proton NMR spectra whose empirical formula is C8H10:
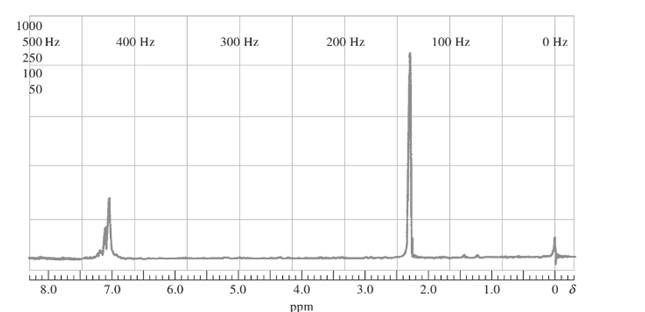
Concept introduction:
Proton NMR is application of nuclear magnetic resonance with respect to 1H nuclei in molecules in order to determine structure. Protons that are in the same magnetic environment are considered as chemically equivalent protons.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Principles of Instrumental Analysis
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


