
Concept explainers
Show by a series of equations how could synthesize each of the following compounds from the indicated starting material and any necessary organic or inorganic reagents:
(a)
(b)
(c)
(d)
(e)
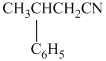
(f) 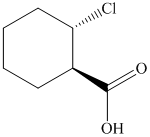 from
from 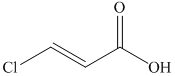
(g)
(h)
(i)
Interpretation:
The way in which each of the given compounds can be synthesized from the indicated starting material and any necessary organic or inorganic reagents is to be shown by using a series of equations.
Concept Introduction:
Hydroboration reaction is a two-step reaction that involves conversion of an alkene into alcohol. This type of reaction follows anti-Markovnikov's rule.In acid-catalyzed dehydration, a saturated compound is converted to an unsaturated compound with the removal of a water molecule in the presence of an acid catalyst.
Grignard reagent is prepared by the reaction of alkyl or aryl bromide with magnesium metal in the presence of ether.
Thionyl chloride
Lithium aluminium hydride is a strong reducing agent. The reduction of carboxylic acid by
Oxidation reaction involves increase in the
Answer to Problem 18P
Solution:
a)The reaction that shows the preparation of
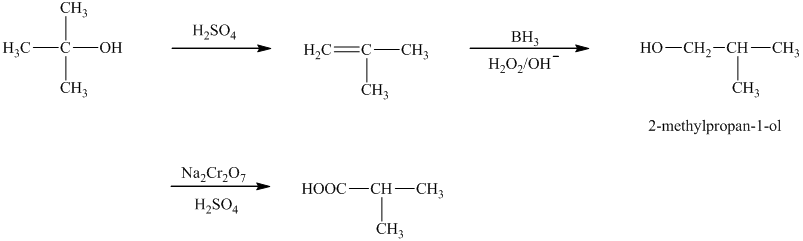
b)The reaction that shows the preparation of
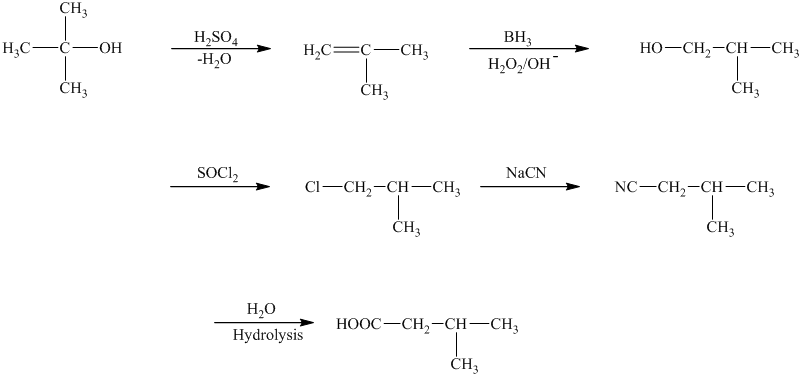
c)The reaction that shows the preparation of
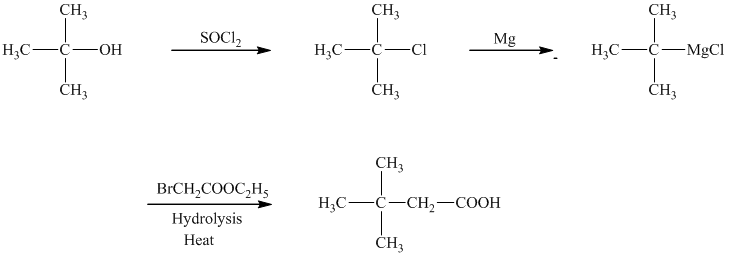
d)The reaction that shows the preparation of
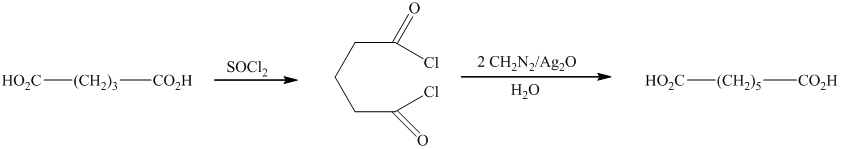
e) The reaction that shows the preparation of
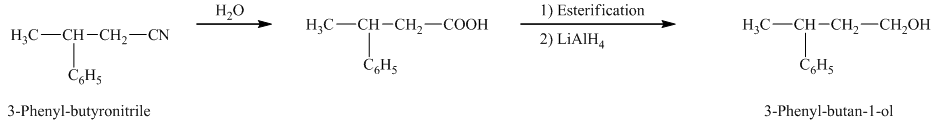
f) The reaction that shows the preparation of
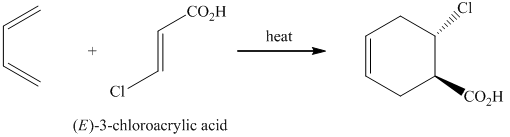
g) The reaction that shows the preparation of
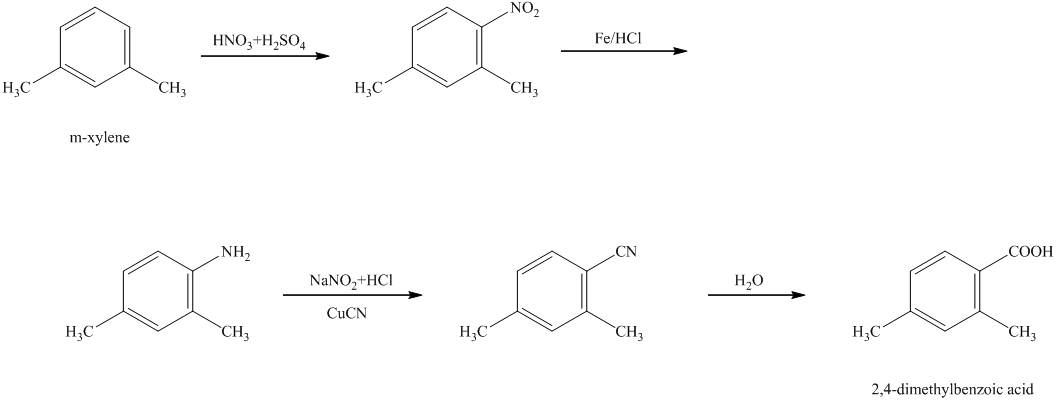
h) The reaction that shows the preparation of
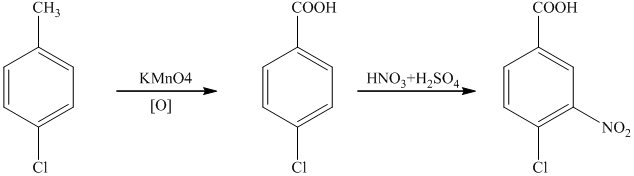
i) The reaction that shows the preparation of
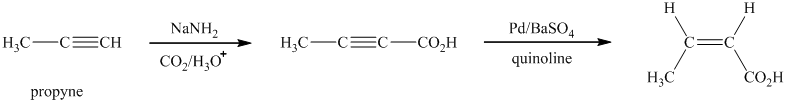
Explanation of Solution
a)
In the synthesis of
Thus, the reaction that shows the preparation of

b)
The reaction that shows the preparation of
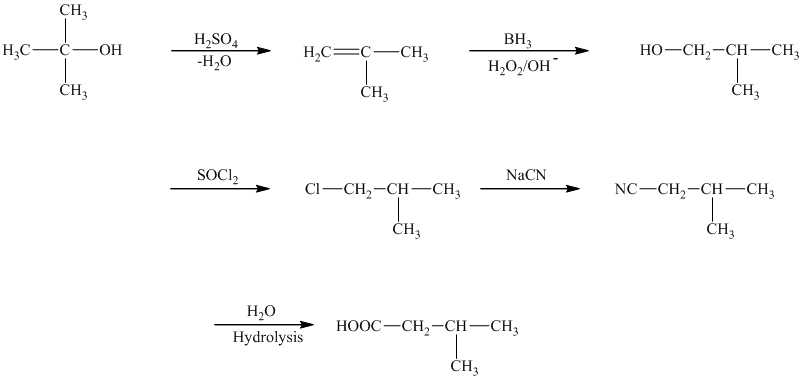
The first step of the required synthesis is the reaction of acid with the given alcohol. This step is the dehydration of alcohol. In the next step, hydroboration of alkene is done, which is then followed by hydrolysis. This results in the formation of
Thus, the required acid is synthesized.
c)
In the required synthesis, the first step is the reaction of the given alcohol with
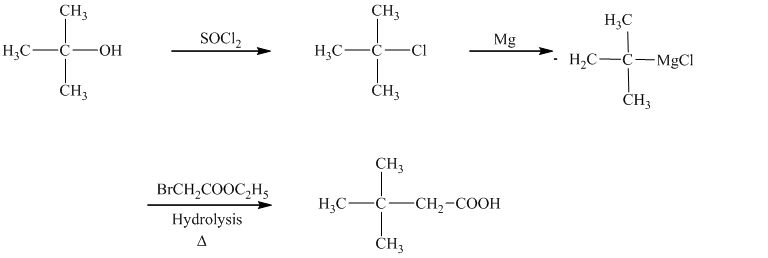
d)
In this synthesis, the first step is the reaction of the given carboxylic acid with thionyl chloride to form acyl chloride. In the next step, acyl chloride reacts with diazomethane to form diazoketones. In the last step of the synthesis reaction, the diazoketones give the final product in the presence of metal catalyst and water. The given reaction is an example of Arndt-Eistert synthesis.
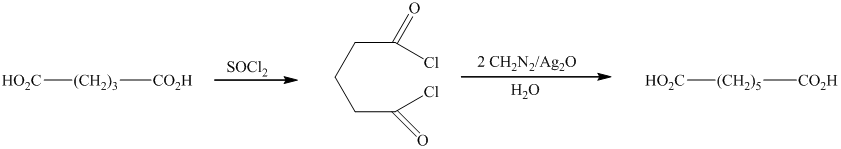
Therefore, the required product is synthesized.
e) 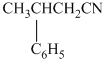
In the required synthesis, the first step is the hydrolysis of
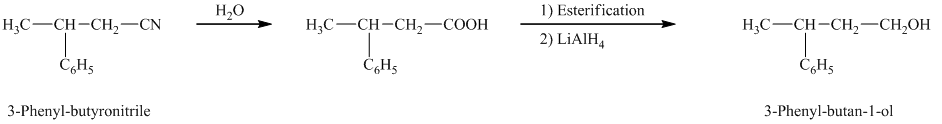
Therefore, the required product is synthesized.
f) 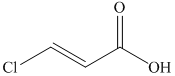
The reaction of
Therefore, the required product is synthesized.
g)
In the required synthesis, the first step is the nitration of
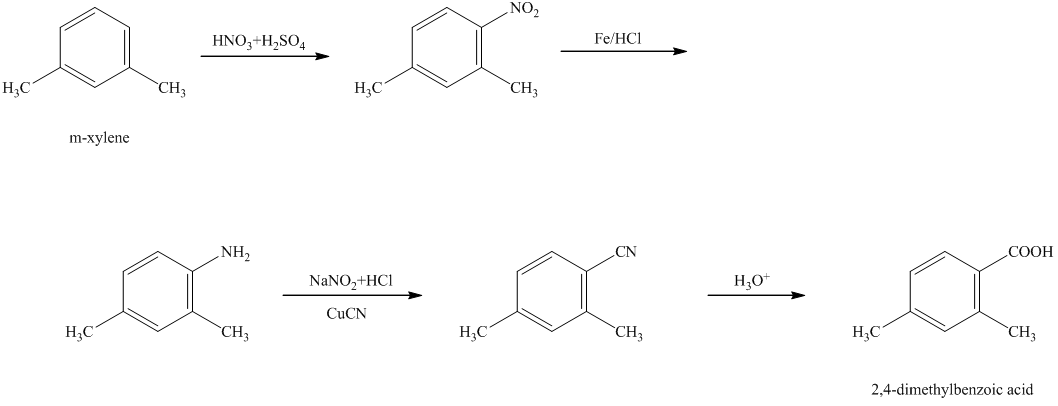
Thus, the required product was synthesized.
h)
In the synthesis of
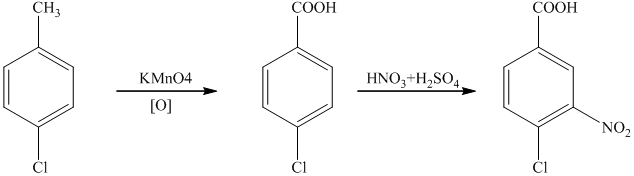
Therefore, the given compound was synthesized.
i)
Sodium amide is a strong base and acts as s strong nucleophile.The reaction that shows the preparation of
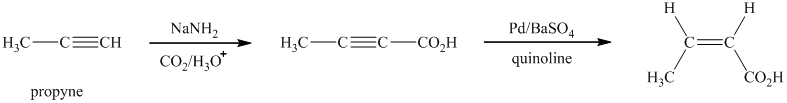
In the above synthesis, the first step is the reaction of
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry - Standalone book
- b. CH3 H3C 'N' H3C CH3 CN Ph 1. OH N 2. H2O2, Pyridinearrow_forwardFor each of the Followin, moleaks draw all OF The Resonance contributing stuluctures and compare these three molecules in terms of Resonance stabilization 1-C-1 a. b. H A-C+ О 112-1 C. F-C-F Farrow_forwarda. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forward
- Question 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forwardIndicate what the Luther equation is used for?arrow_forward
- Indicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

