
EBK ORGANIC CHEMISTRY
9th Edition
ISBN: 8220100591310
Author: McMurry
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18.SE, Problem 70GP
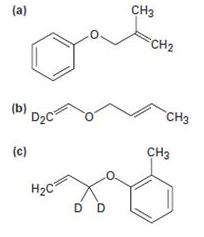
Predict the product(s) if the starting materials below underwent a Claisen rearrangement. Draw arrows to illustrate the rearrangement of electrons.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show reaction mechanism. Don't give Ai generated solution
Describe some isomerism that carboranes have.
Indicate an isomerism that carboranes present.
Chapter 18 Solutions
EBK ORGANIC CHEMISTRY
Ch. 18.1 - Name the following ethers:Ch. 18.2 - Why do you suppose only symmetrical ethers are...Ch. 18.2 - How would you prepare the following ethers using a...Ch. 18.2 - Review the mechanism of oxymercuration shown in...Ch. 18.2 - How would you prepare the following ethers? Use...Ch. 18.2 - Prob. 6PCh. 18.3 - Prob. 7PCh. 18.3 - Write the mechanism of the acid-induced cleavage...Ch. 18.3 - Why are HI and HBr more effective than HCl in...Ch. 18.4 - What product would you expect from Claisen...
Ch. 18.5 - Prob. 11PCh. 18.6 - Predict the major product of each of the following...Ch. 18.6 - Prob. 13PCh. 18.6 - Predict the major product of the following...Ch. 18.7 - 15-Crown-5 and 12-crown-4 ethers complex Na+ and...Ch. 18.8 - Prob. 16PCh. 18.8 - 2-Butene-l-thiol is one component of skunk spray....Ch. 18.9 - The 1H NMR spectrum shown is that of a cyclic...Ch. 18.SE - Give IUPAC names for the following compounds...Ch. 18.SE - Show the product, including stereochemistry, that...Ch. 18.SE - Prob. 21VCCh. 18.SE - Treatment of the following alkene with a...Ch. 18.SE - Prob. 23MPCh. 18.SE - Prob. 24MPCh. 18.SE - Predict the product(s) and provide the mechanism...Ch. 18.SE - The alkoxymercuration of alkenes involves the...Ch. 18.SE - Predict the product(s) and provide the mechanism...Ch. 18.SE - Predict the product(s) and provide the mechanism...Ch. 18.SE - Prob. 29MPCh. 18.SE - Ethers undergo an acid-catalyzed cleavage reaction...Ch. 18.SE - Treatment of 1, 1-diphenyl-l, 2-epoxyethane with...Ch. 18.SE - Fluoxetine, a heavily prescribed antidepressant...Ch. 18.SE - When 2-methyl-2, 5-pentanediol is treated with...Ch. 18.SE - Prob. 34MPCh. 18.SE - Prob. 35MPCh. 18.SE - Aldehydes and ketones undergo acid-catalyzed...Ch. 18.SE - Propose a mechanism to account for the following...Ch. 18.SE - Prob. 38APCh. 18.SE - Prob. 39APCh. 18.SE - How would you prepare the following ethers?Ch. 18.SE - Prob. 41APCh. 18.SE - tert-Butyl ethers can be prepared by the reaction...Ch. 18.SE - Treatment of trans-2-chlorocyclohexanol with NaOH...Ch. 18.SE - Predict the products of the following ether...Ch. 18.SE - Prob. 45APCh. 18.SE - Prob. 46APCh. 18.SE - Write the mechanism of the hydrolysis of cis-5,...Ch. 18.SE - Prob. 48APCh. 18.SE - Acid-catalyzed hydrolysis of a 1,...Ch. 18.SE - Prob. 50APCh. 18.SE - Epoxides are reduced by treatment with lithium...Ch. 18.SE - Prob. 52APCh. 18.SE - The red fox (Vulpes vulpes) uses a chemical...Ch. 18.SE - Anethole, C10H12O, a major constituent of the oil...Ch. 18.SE - Propose structures for compounds that have the...Ch. 18.SE - Prob. 56GPCh. 18.SE - How would you synthesize anethole (Problem 18-54)...Ch. 18.SE - How could you prepare benzyl phenyl ether from...Ch. 18.SE - Meerwein's reagent, triethyloxonium...Ch. 18.SE - Prob. 60GPCh. 18.SE - Prob. 61GPCh. 18.SE - The Zeisel method is an old analytical procedure...Ch. 18.SE - Prob. 63GPCh. 18.SE - Prob. 64GPCh. 18.SE - Prob. 65GPCh. 18.SE - Identify the reagents a-e in the following scheme:Ch. 18.SE - Propose structures for compounds that have the...Ch. 18.SE - Prob. 68GPCh. 18.SE - Prob. 69GPCh. 18.SE - Predict the product(s) if the starting materials...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Transmitance 3. Which one of the following compounds corresponds to this IR spectrum? Point out the absorption band(s) that helped you decide. OH H3C OH H₂C CH3 H3C CH3 H3C INFRARED SPECTRUM 0.8- 0.6 0.4- 0.2 3000 2000 1000 Wavenumber (cm-1) 4. Consider this compound: H3C On the structure above, label the different types of H's as A, B, C, etc. In table form, list the labeled signals, and for each one state the number of hydrogens, their shifts, and the splitting you would observe for these hydrogens in the ¹H NMR spectrum. Label # of hydrogens splitting Shift (2)arrow_forwardNonearrow_forwardDraw the Lewis structure of C2H4Oarrow_forward
- a) 5. Circle all acidic (and anticoplanar to the Leaving group) protons in the following molecules, Solve these elimination reactions, and identify the major and minor products where appropriate: 20 points + NaOCH3 Br (2 productarrow_forwardNonearrow_forwardDr. Mendel asked his BIOL 260 class what their height was and what their parent's heights were. He plotted that data in the graph below to determine if height was a heritable trait. A. Is height a heritable trait? If yes, what is the heritability value? (2 pts) B. If the phenotypic variation is 30, what is the variation due to additive alleles? (2 pts) Offspring Height (Inches) 75 67.5 60 52.5 y = 0.9264x + 4.8519 55 60 65 MidParent Height (Inches) 70 75 12pt v V Paragraph B IUA > AT2 v Varrow_forward
- Experiment: Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below. Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization. Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C Sample C-Cool the third sample in an ice-bath to 0-2 °C Results: weight after recrystalization and melting point temp. A=0.624g,102-115° B=0.765g, 80-105° C=1.135g, 77-108 What is the percent yield of A,B, and C.arrow_forwardRel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forwardIllustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License