
Concept explainers
a)
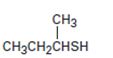
Interpretation:
The name of the compound given is to be stated.
Concept introduction:
Thioalcohols are named as derivatives of the parent
To give:
The name of the compound shown.
b)
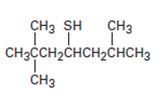
Interpretation:
The name of the compound given is to be stated.
Concept introduction:
Thioalcohols are named as derivatives of the parent alkane using the suffix –al. The longest carbon chain containing the thiol group is chosen and the parent name is derived by replacing the ending –e with –thiol. The alkane chain is numbered beginning at the end nearer to the thiol group. The substituents are numbered according to their position on the chain. The name is written listing the substituents in the alphabetical order and indicating the position to which –SH is bonded.
To give:
The name of the compound shown.
c)
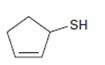
Interpretation:
The name of the compound given is to be stated.
Concept introduction:
In naming cyclic thioalcohols the parent name is derived from the cycloalkene ring by replacing –e of the cycloalkene with –thiol. The ring is numbered from the carbon with –SH in such a way that lowest number possible is given to the other
To give:
The name of the compound shown.
d)
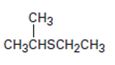
Interpretation:
The name of the compound given is to be stated.
Concept introduction:
Simple sulfides are with no other functional groups are named by identifying the two organic substituents and adding the word sulfide. If other functional groups are present, the sulfide part is considered as an alkylthio substituent.
To give:
The name of the sulfide shown.
e)
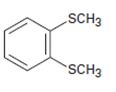
Interpretation:
The name of the compound given is to be stated.
Concept introduction:
Simple sulfides are with no other functional groups are named by identifying the two organic substituents and adding the word sulfide. If other functional groups are present, the sulfide part is considered as an alkylthio substituent.
To give:
The name of the disulfide shown.
f)
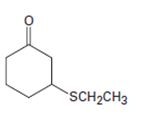
Interpretation:
The name of the compound given is to be stated.
Concept introduction:
Simple sulfides are with no other functional groups are named by identifying the two organic substituents and adding the word sulfide. If other functional groups are present, the sulfide part is considered as an alkylthio substituent.
To give:
The name of the compound shown.
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





