
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
11th Edition
ISBN: 9781305705159
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18.5, Problem 18.3P
Interpretation Introduction
(a)
Interpretation:
The given Fischer esterification should be completed.
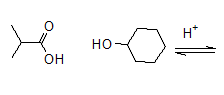
Concept Introduction:
Interpretation Introduction
(b)
Interpretation:
The given Fischer esterification should be completed.
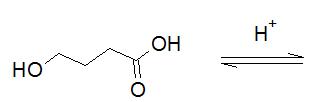
Concept Introduction:
Carboxylic acid group reacts with hydroxyl group to give ester and water.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
42. Which of the following halogenated compounds can be used successfully to prepare a Grignard reagent for
alcohol synthesis by subsequent reaction with an aldehyde or ketone? Which ones cannot and why?
H3C CH3
a.
Br
H OH
b.
Cl
C.
I H
H
d. Cl
e.
H
OCH3
Br
H
For each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check
the appropriate box.
Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below.
Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions
- just focus on the first stable product you expect to form in solution.
?
Will the first
MgBr
product that forms in this reaction
create a new CC bond?
olo
?
OH
جمله
O Yes
Ⓒ No
MgCl
?
Will the first product that forms in this reaction
create a new CC bond?
Click and drag to start drawing a
structure.
Yes
No
X
☐ :
☐
टे
PH
Assign all the protons
Chapter 18 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 18.2 - Prob. 18.1PCh. 18.5 - Prob. 18.2PCh. 18.5 - Prob. 18.3PCh. 18 - 18-4 Answer true or false. (a) The functional...Ch. 18 - Prob. 18.5PCh. 18 - 18-6 Name and draw structural formulas for the...Ch. 18 - 18-7 Write the IUPAC name for each carboxylic...Ch. 18 - 18-8 Write the IUPAC name for each carboxylic...Ch. 18 - Prob. 18.9PCh. 18 - Prob. 18.10P
Ch. 18 - Prob. 18.11PCh. 18 - Prob. 18.12PCh. 18 - Prob. 18.13PCh. 18 - 18-14 Answer true or false. (a) Carboxylic acids...Ch. 18 - 18-15 Draw a structural formula for the dimer...Ch. 18 - 18-16 Propanedioic (malonic) acid forms an...Ch. 18 - 18-17 Hexanoic (caproic) acid has a solubility in...Ch. 18 - 18-18 Propanoic acid and methyl acetate are...Ch. 18 - 18-19 The following compounds have approximately...Ch. 18 - Prob. 18.20PCh. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - 18-23 Characterize the structural features...Ch. 18 - Prob. 18.24PCh. 18 - Prob. 18.25PCh. 18 - 18-26 Answer true or false. (a) Carboxylic acids...Ch. 18 - Prob. 18.27PCh. 18 - 18-28 Arrange these compounds in order of...Ch. 18 - 18-29 Complete the equations for these acid—base...Ch. 18 - 18-30 Complete the equations for these acid-base...Ch. 18 - 18-31 Formic acid is one of the components...Ch. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Prob. 18.36PCh. 18 - Prob. 18.37PCh. 18 - 18-38 Which is the stronger base: CH3CH2NH2 or...Ch. 18 - Prob. 18.39PCh. 18 - Prob. 18.40PCh. 18 - 18-41 Complete these examples of Fischer...Ch. 18 - Prob. 18.42PCh. 18 - Prob. 18.43PCh. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - 18-46 Procaine (its hydrochloride salt is marketed...Ch. 18 - 18-47 Methylparaben and propylparaben are used as...Ch. 18 - 18-48 4-Aminobenzoic acid is prepared from benzoic...Ch. 18 - Prob. 18.49PCh. 18 - Prob. 18.50PCh. 18 - Prob. 18.51PCh. 18 - Prob. 18.52PCh. 18 - Prob. 18.53PCh. 18 - Prob. 18.54PCh. 18 - Prob. 18.55P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9 7 8 C 9 8 200 190 B 5 A -197.72 9 8 7 15 4 3 0: ང་ 200 190 180 147.52 134.98 170 160 150 140 130 120 110 100 90 90 OH 10 4 3 1 2 -143.04 140. 180 170 160 150 140 130 120 110 100 90 CI 3 5 1 2 141.89 140.07 200 190 180 170 160 150 140 130 120 110 100 ៖- 90 129. 126.25 80 70 60 -60 50 40 10 125.19 -129.21 80 70 3.0 20 20 -8 60 50 10 ppm -20 40 128.31 80 80 70 60 50 40 40 -70.27 3.0 20 10 ppm 00˚0-- 77.17 30 20 20 -45.36 10 ppm -0.00 26.48 22.32 ―30.10 ―-0.00arrow_forwardAssign all the carbonsarrow_forwardC 5 4 3 CI 2 the Righ B A 5 4 3 The Lich. OH 10 4 5 3 1 LOOP- -147.52 T 77.17 -45.36 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 ppm B -126.25 77.03 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 ppm 200 190 180 170 160 150 140 130 120 110 100 90 80 TO LL <-50.00 70 60 50 40 30 20 10 ppm 45.06 30.18 -26.45 22.36 --0.00 45.07 7.5 1.93 2.05 -30.24 -22.36 C A 7 8 5 ° 4 3 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 8 5 4 3 ཡི་ OH 10 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 5 4 3 2 that th 7 I 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 115 2.21 4.00 1.0 ppm 6.96 2.76 5.01 1.0 ppm 6.30 1.00arrow_forward
- Curved arrows were used to generate the significant resonance structure and labeled the most significant contribute. What are the errors in these resonance mechanisms. Draw out the correct resonance mechanisms with an brief explanation.arrow_forwardWhat are the: нсе * Moles of Hice while given: a) 10.0 ml 2.7M ? 6) 10.ome 12M ?arrow_forwardYou are asked to use curved arrows to generate the significant resonance structures for the following series of compounds and to label the most significant contributor. Identify the errors that would occur if you do not expand the Lewis structures or double-check the mechanisms. Also provide the correct answers.arrow_forward
- how to get limiting reactant and % yield based off this data Compound Mass 6) Volume(mL Ben zaphone-5008 ne Acetic Acid 1. Sam L 2-propanot 8.00 Benzopin- a col 030445 Benzopin a Colone 0.06743 Results Compound Melting Point (°c) Benzopin acol 172°c - 175.8 °c Benzoping to lone 1797-180.9arrow_forwardAssign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
