
ORGANIC CHEMISTRY, WITH SOL. MAN/ STUDY
3rd Edition
ISBN: 9781119477617
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18, Problem 89IP
Interpretation Introduction
Interpretation:
Major product of the given reaction has to be identified from the given option.

Concept Introduction:
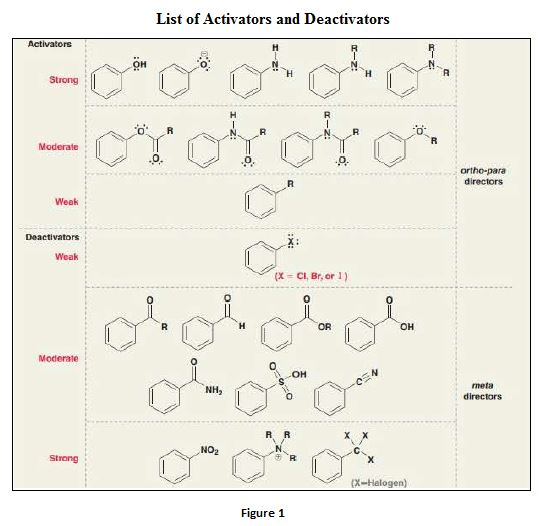
- Electrophilic substitution reaction is a type of reaction in which a particular group or atom in a compound is replaced by electrophile. An electrophile is a species that is deficient of electrons
- Benzene is an electron rich
aromatic compound and undergoes electrophilic substitution reactions. - The position that the electrophile occupies in the ring system depends on various factors like – presence of substituents, activation and deactivation of the ring etc.
- The pi electrons of the aromatic system must be available freely for the reaction to occur. Further, the aromatic ring must not have many bulk substituents on it to ease the any type of electrophilic substitution reaction. Presence of many bulk substituents hinders the ability of the delocalizing pi electrons to bond with the electrophile.
Deactivators are electron withdrawing groups attached to the benzenes that have either positive charge or an atom with high electronegativity. They are meta directors.
Activators are electron donating groups attached to the benzenes that have either electron density that is able to push into benzene ring or a lone pair of electrons. They are ortho–para directing.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major organic product(s) of the following reactions. Indicate which of the following mechanisms is in operation: SN1, SN2, E1, or E2.
(c)
(4pts)
Mechanism:
heat
(E1)
CH3OH
+
1.5pts each
_E1 _ (1pt)
Br
CH3OH
(d)
(4pts)
Mechanism:
SN1
(1pt)
(e)
(3pts)
1111 I
H
10
Ill!!
H
LDA
THF (solvent)
Mechanism: E2
(1pt)
NC
(f)
Bri!!!!!
CH3
NaCN
(3pts)
acetone
Mechanism: SN2
(1pt)
(SN1)
-OCH3
OCH3
1.5pts each
2pts for either product
1pt if incorrect
stereochemistry
H
Br
(g)
“,、
(3pts)
H
CH3OH
+21
Mechanism:
SN2
(1pt)
H
CH3
2pts
1pt if incorrect
stereochemistry
H
2pts
1pt if incorrect
stereochemistry
A mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixture
Chapter 18 Solutions
ORGANIC CHEMISTRY, WITH SOL. MAN/ STUDY
Ch. 18.2 - Prob. 1CCCh. 18.3 - Prob. 2CCCh. 18.3 - Prob. 3CCCh. 18.4 - Prob. 4CCCh. 18.5 - Prob. 5CCCh. 18.5 - Prob. 6CCCh. 18.5 - Prob. 7CCCh. 18.6 - Prob. 8CCCh. 18.6 - Prob. 9CCCh. 18.6 - Prob. 10CC
Ch. 18.7 - Prob. 11CCCh. 18.7 - Prob. 12CCCh. 18.8 - Prob. 13CCCh. 18.9 - Prob. 14CCCh. 18.9 - Prob. 15CCCh. 18.10 - Prob. 1LTSCh. 18.10 - Prob. 16PTSCh. 18.11 - Prob. 2LTSCh. 18.11 - Prob. 18PTSCh. 18.11 - Prob. 19ATSCh. 18.11 - Prob. 3LTSCh. 18.11 - Prob. 20PTSCh. 18.11 - Prob. 21ATSCh. 18.11 - Prob. 4LTSCh. 18.11 - Prob. 22PTSCh. 18.11 - Prob. 23ATSCh. 18.12 - Prob. 24CCCh. 18.12 - Prob. 25CCCh. 18.12 - Prob. 5LTSCh. 18.12 - Prob. 26PTSCh. 18.12 - 2-Nitroaniline has been used as a precursor in the...Ch. 18.12 - Prob. 6LTSCh. 18.12 - Prob. 28PTSCh. 18.12 - Prob. 29ATSCh. 18.13 - Prob. 30CCCh. 18.13 - Prob. 31CCCh. 18.13 - Prob. 32CCCh. 18.14 - Prob. 33CCCh. 18.14 - Prob. 34CCCh. 18.15 - Prob. 7LTSCh. 18.15 - Prob. 35PTSCh. 18.15 - Prob. 36PTSCh. 18 - Prob. 38PPCh. 18 - Prob. 39PPCh. 18 - Prob. 40PPCh. 18 - Prob. 41PPCh. 18 - Prob. 42PPCh. 18 - Prob. 43PPCh. 18 - Prob. 44PPCh. 18 - Prob. 45PPCh. 18 - Prob. 46PPCh. 18 - Prob. 47PPCh. 18 - Prob. 48PPCh. 18 - Prob. 49PPCh. 18 - Prob. 50PPCh. 18 - Prob. 51PPCh. 18 - Prob. 52PPCh. 18 - Prob. 53PPCh. 18 - Prob. 54PPCh. 18 - Prob. 55PPCh. 18 - Prob. 56PPCh. 18 - Prob. 57PPCh. 18 - Prob. 58PPCh. 18 - Prob. 59PPCh. 18 - Prob. 60PPCh. 18 - Prob. 61PPCh. 18 - Prob. 62PPCh. 18 - Prob. 63PPCh. 18 - Prob. 64PPCh. 18 - When 2,4-dibromo-3-methyltolene is treated with...Ch. 18 - Prob. 66PPCh. 18 - Prob. 67PPCh. 18 - Prob. 68PPCh. 18 - Prob. 69PPCh. 18 - Prob. 70PPCh. 18 - Prob. 71PPCh. 18 - Prob. 72PPCh. 18 - Prob. 74IPCh. 18 - Prob. 75IPCh. 18 - Prob. 76IPCh. 18 - Prob. 77IPCh. 18 - Prob. 78IPCh. 18 - Prob. 79IPCh. 18 - Prob. 80IPCh. 18 - Prob. 81IPCh. 18 - Prob. 82IPCh. 18 - Prob. 83IPCh. 18 - Prob. 84IPCh. 18 - Prob. 85IPCh. 18 - Prob. 86IPCh. 18 - Prob. 87IPCh. 18 - Prob. 88IPCh. 18 - Prob. 89IPCh. 18 - Prob. 90IPCh. 18 - Prob. 91CPCh. 18 - Prob. 92CPCh. 18 - In the following reaction, iodine monochloride...Ch. 18 - Prob. 94CPCh. 18 - The following synthesis was developed in an...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY