
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics, Books a la Carte Edition; Student Workbook for Physics for Scientists ... eText -- ValuePack Access Card (4th Edition)
4th Edition
ISBN: 9780134564234
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 58EAP
The 50 kg circular piston shown
in FIGURE P18.58 floats on 0. 12 mol
of compressed air.
a. What is the piston height h if
the temperature is 30oC?
b. How far does the piston move
if the temperature is increased
by 100oC?
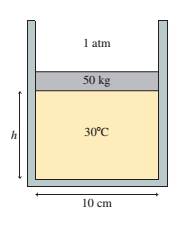
FIGURE P18.58
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Fresnel lens: You would like to design a 25 mm diameter blazed Fresnel zone plate with a first-order power of
+1.5 diopters. What is the lithography requirement (resolution required) for making this lens that is designed
for 550 nm? Express your answer in units of μm to one decimal point.
Fresnel lens: What would the power of the first diffracted order of this lens be at wavelength of 400 nm?
Express your answer in diopters to one decimal point.
Eye: A person with myopic eyes has a far point of 15 cm. What power contact lenses does she need to correct
her version to a standard far point at infinity? Give your answer in diopter to one decimal point.
Paraxial design of a field flattener. Imagine your optical system has Petzal curvature of the field with radius
p. In Module 1 of Course 1, a homework problem asked you to derive the paraxial focus shift along the axis
when a slab of glass was inserted in a converging cone of rays. Find or re-derive that result, then use it to
calculate the paraxial radius of curvature of a field flattener of refractive index n that will correct the observed
Petzval. Assume that the side of the flattener facing the image plane is plano. What is the required radius of
the plano-convex field flattener? (p written as rho )
3.37(a) Five free electrons exist in a three-dimensional infinite potential well with all three widths equal to \( a = 12 \, \text{Å} \). Determine the Fermi energy level at \( T = 0 \, \text{K} \). (b) Repeat part (a) for 13 electrons.
Book: Semiconductor Physics and Devices 4th ed, NeamanChapter-3Please expert answer only. don't give gpt-generated answers, & please clear the concept of quantum states for determining nx, ny, nz to determine E, as I don't have much idea about that topic.
Chapter 18 Solutions
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics, Books a la Carte Edition; Student Workbook for Physics for Scientists ... eText -- ValuePack Access Card (4th Edition)
Ch. 18 - Prob. 1CQCh. 18 - Prob. 2CQCh. 18 - Prob. 3CQCh. 18 - Prob. 4CQCh. 18 - Prob. 5CQCh. 18 - Prob. 6CQCh. 18 - Prob. 7CQCh. 18 - Prob. 8CQCh. 18 - Prob. 9CQCh. 18 - A gas undergoes the process shown in FIGURE...
Ch. 18 - Prob. 11CQCh. 18 - Prob. 12CQCh. 18 - Prob. 1EAPCh. 18 - Prob. 2EAPCh. 18 - What is the diameter of a copper sphere that has...Ch. 18 - Prob. 4EAPCh. 18 - Prob. 5EAPCh. 18 - How many atoms are in a 2.0 cm × 2.0 cm × 2.0 cm...Ch. 18 - Prob. 7EAPCh. 18 - An element in its solid phase has mass density...Ch. 18 - .0 mol of gold is shaped into a sphere. What is...Ch. 18 - What volume of aluminum has the same number of...Ch. 18 - Prob. 11EAPCh. 18 - Prob. 12EAPCh. 18 - Prob. 13EAPCh. 18 - A concrete bridge is built of 325-cm-long concrete...Ch. 18 - A surveyor has a steel measuring tape that is...Ch. 18 - Two students each build a piece of scientific...Ch. 18 - Prob. 17EAPCh. 18 -
18. What is the temperature in °F and the...Ch. 18 - Prob. 19EAPCh. 18 - .0 mol of gas at a temperature of -120°C fills a...Ch. 18 - Prob. 21EAPCh. 18 - Prob. 22EAPCh. 18 - Prob. 23EAPCh. 18 - Prob. 24EAPCh. 18 - Prob. 25EAPCh. 18 - Prob. 26EAPCh. 18 - Prob. 27EAPCh. 18 - Prob. 28EAPCh. 18 - A rigid, hollow sphere is submerged in boiling...Ch. 18 -
30. A rigid container holds hydrogen gas at a...Ch. 18 - Prob. 31EAPCh. 18 - Prob. 32EAPCh. 18 - Prob. 33EAPCh. 18 - Prob. 34EAPCh. 18 - Prob. 35EAPCh. 18 - Prob. 36EAPCh. 18 - Prob. 37EAPCh. 18 - .0050 mol of gas undergoes the process 1 2 3...Ch. 18 - Prob. 39EAPCh. 18 - Prob. 40EAPCh. 18 - Prob. 41EAPCh. 18 - Prob. 42EAPCh. 18 - Prob. 43EAPCh. 18 - A 15°C, 2.0-cm-diameter aluminum bar just barely...Ch. 18 - Prob. 45EAPCh. 18 - Prob. 46EAPCh. 18 - Prob. 47EAPCh. 18 - Prob. 48EAPCh. 18 - Prob. 49EAPCh. 18 - The 3.0-m-long pipe in FIGURE P18.50 is closed at...Ch. 18 - Prob. 51EAPCh. 18 - An electric generating plant boils water to...Ch. 18 - Prob. 53EAPCh. 18 - The air temperature and pressure in a laboratory...Ch. 18 - Prob. 55EAPCh. 18 - The mercury manometer shown in FIGURE P18.56 is...Ch. 18 - Prob. 57EAPCh. 18 - The 50 kg circular piston shown in FIGURE P18.58...Ch. 18 - Prob. 59EAPCh. 18 - .0 g of helium gas follows the process 1? 2 ?3...Ch. 18 - Prob. 61EAPCh. 18 - 62. FIGURE P18.62 shows two different processes...Ch. 18 - Prob. 63EAPCh. 18 - Prob. 64EAPCh. 18 - Prob. 65EAPCh. 18 - Prob. 66EAPCh. 18 - Prob. 67EAPCh. 18 - Prob. 68EAPCh. 18 - Prob. 69EAPCh. 18 - Prob. 70EAPCh. 18 - Prob. 71EAPCh. 18 - The cylinder in FIGURE CP18.72 has a moveable...Ch. 18 - Containers A and B in FIGURE CP18.73 hold the same...Ch. 18 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 3.37(a) Five free electrons exist in a three-dimensional infinite potential well with all three widths equal to \( a = 12 \, \text{Å} \). Determine the Fermi energy level at \( T = 0 \, \text{K} \). (b) Repeat part (a) for 13 electrons. Book: Semiconductor Physics and Devices 4th ed, NeamanChapter-3Please expert answer only. don't give gpt-generated answers, & please clear the concept of quantum states for determining nx, ny, nz to determine E, as I don't have much idea about that topic.arrow_forwardNo chatgpt pls will upvotearrow_forwardUse the following information to answer the next question. Two mirrors meet an angle, a, of 105°. A ray of light is incident upon mirror A at an angle, i, of 42°. The ray of light reflects off mirror B and then enters water, as shown below: Incident ray at A Note: This diagram is not to scale. a Air (n = 1.00) Water (n = 1.34) 1) Determine the angle of refraction of the ray of light in the water. Barrow_forward
- Hi can u please solvearrow_forward6. Bending a lens in OpticStudio or OSLO. In either package, create a BK7 singlet lens of 10 mm semi-diameter and with 10 mm thickness. Set the wavelength to the (default) 0.55 microns and a single on-axis field point at infinite object distance. Set the image distance to 200 mm. Make the first surface the stop insure that the lens is fully filled (that is, that the entrance beam has a radius of 10 mm). Use the lens-maker's equation to calculate initial glass curvatures assuming you want a symmetric, bi-convex lens with an effective focal length of 200 mm. Get this working and examine the RMS spot size using the "Text" tab of the Spot Diagram analysis tab (OpticStudio) or the Spd command of the text widnow (OSLO). You should find the lens is far from diffraction limited, with a spot size of more than 100 microns. Now let's optimize this lens. In OpticStudio, create a default merit function optimizing on spot size.Then insert one extra line at the top of the merit function. Assign the…arrow_forwardNo chatgpt pls will upvote Already got wrong chatgpt answer .arrow_forward
- Use the following information to answer the next question. Two mirrors meet an angle, a, of 105°. A ray of light is incident upon mirror A at an angle, i, of 42°. The ray of light reflects off mirror B and then enters water, as shown below: A Incident ray at A Note: This diagram is not to scale. Air (n = 1.00) Water (n = 1.34) Barrow_forwardUse the following information to answer the next question. Two mirrors meet an angle, a, of 105°. A ray of light is incident upon mirror A at an angle, i, of 42°. The ray of light reflects off mirror B and then enters water, as shown below: A Incident ray at A Note: This diagram is not to scale. Air (n = 1.00) Water (n = 1.34) Barrow_forwardGood explanation it sure experts solve it.arrow_forward
- No chatgpt pls will upvote Asaparrow_forwardA satellite has a mass of 100kg and is located at 2.00 x 10^6 m above the surface of the earth. a) What is the potential energy associated with the satellite at this loction? b) What is the magnitude of the gravitational force on the satellite?arrow_forwardNo chatgpt pls will upvotearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning


Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY